Similar Questions
Explore conceptually related problems
Recommended Questions
- Half mole of an ideal gas (gamma = 5/3) is taken through the cycle abc...
Text Solution
|
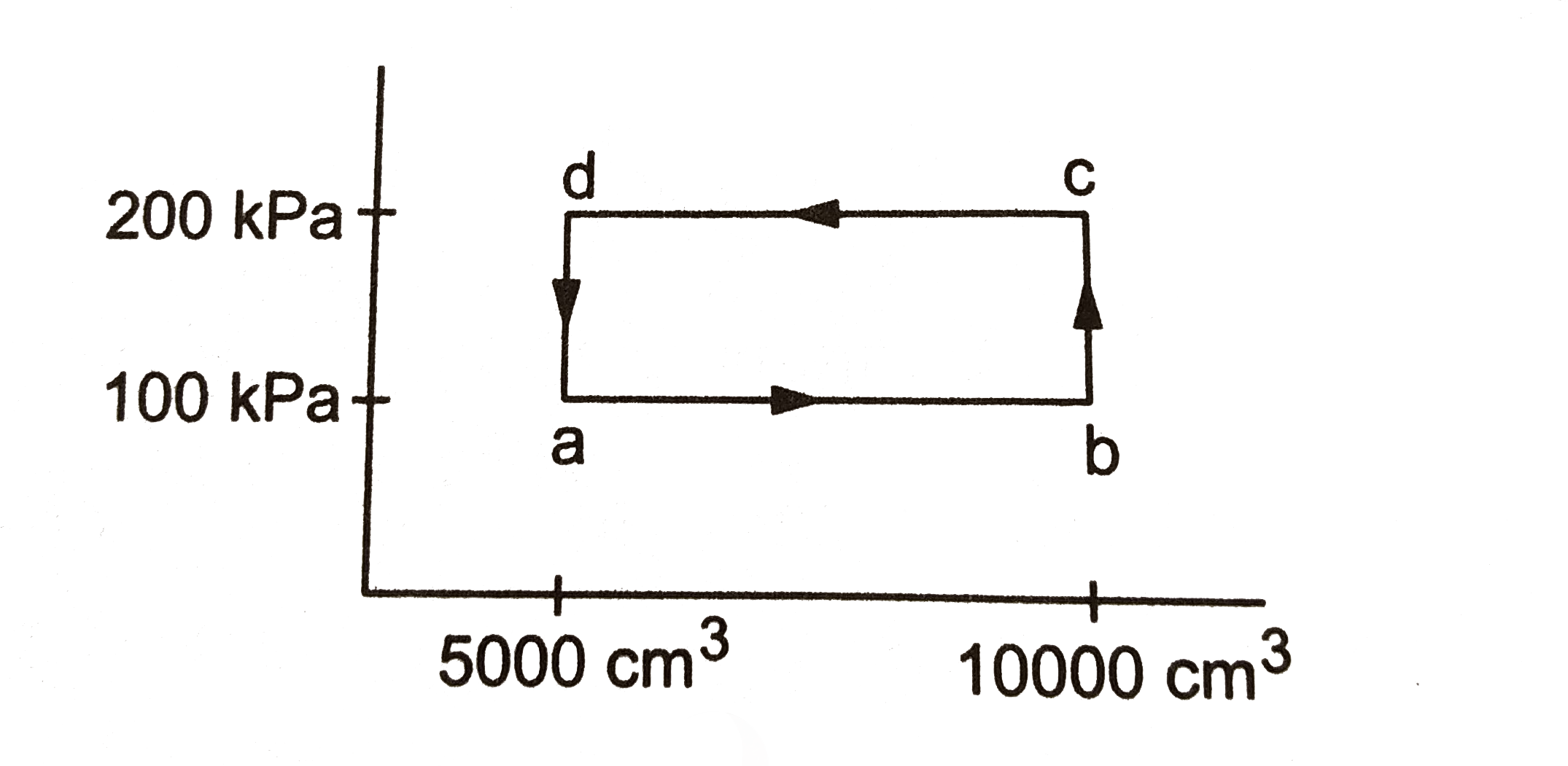
- A thermodynamic system is taken through the cycle abcda during the par...
Text Solution
|
- consider the cyclic process ABCA on a sample of 2.0 mol of an ideal ga...
Text Solution
|
- Half mole of an ideal gas (gamma = 5/3) is taken through the cycle abc...
Text Solution
|
- An ideal gas (gamma = 1.67 ) is taken through the process abc shown in...
Text Solution
|
- In figure, a cyclic process ABCA of 3 moles of an ideal gas is given. ...
Text Solution
|
- In given figure, one mole of an ideal gas (gamma = 7//5) is taken thro...
Text Solution
|
- An ideal gas is taken through the cycle A rarr B rarr C rarr A As show...
Text Solution
|
- A thermodynamic system is taken through the cycle abcda. (a) calculate...
Text Solution
|