Similar Questions
Explore conceptually related problems
Recommended Questions
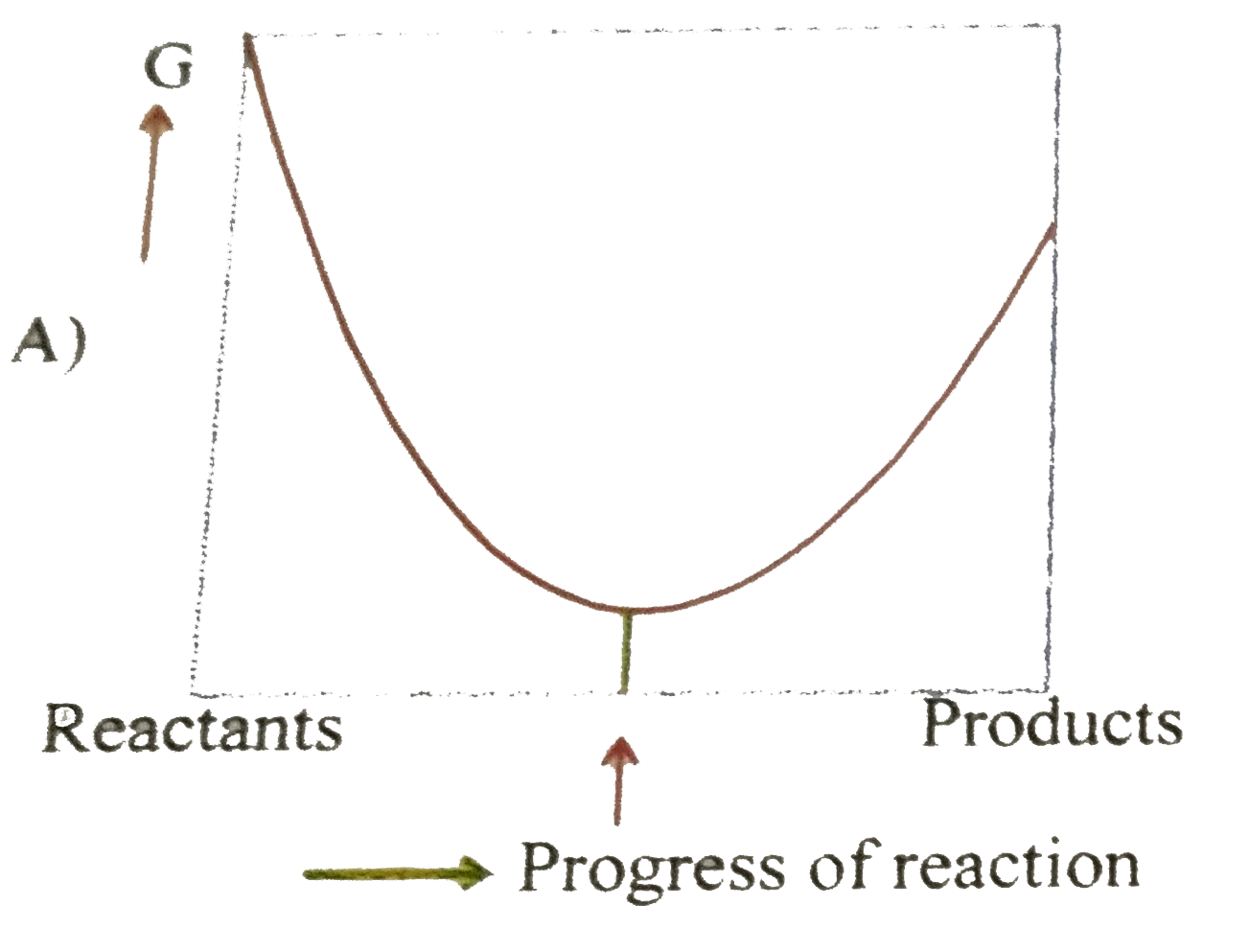
- For a reversible reaction at constant temperature and at constant pres...
Text Solution
|
- The equilibrium constant in a reversible reaction at a given temperatu...
Text Solution
|
- The equilibrium constant in a reversible reaction at given temperature
Text Solution
|
- For a reversible reaction at constant temperature and at constant pres...
Text Solution
|
- For a reversible reaction at constant temperature and at constant pres...
Text Solution
|
- For a reversible reaction at constant temperature and at constant pres...
Text Solution
|
- If the equilibrium constant for a reaction is 4*0 , what will be the e...
Text Solution
|
- किसी दिए गए ताप पर उत्क्रमणीय अभिक्रिया का साम्य स्थिरांक
Text Solution
|
- The gibbs free energy for a reversible reaction at equilibrium is
Text Solution
|