Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
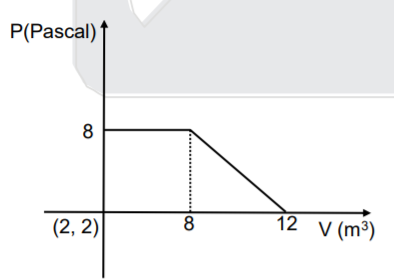
- A gas undergoes expansion according to the following graph. Calculate ...
Text Solution
|
- Obtain an expression for work done by a gas in an isothermal expansion...
Text Solution
|
- The amount of work done by the gas during expansion is greatest by
Text Solution
|
- गैस के समतापी प्रसार में कृत कार्य निर्भर है :
Text Solution
|
- A gas undergoes expansion according to the following graph. Calculate ...
Text Solution
|
- 1 mol of an ideal gas is enclosed in a cylinder fitted with a friction...
Text Solution
|
- What is the work done in free expansion of an ideal gas?
Text Solution
|
- In case of expansion of a gas, would the work done by the gas be posit...
Text Solution
|
- A gas undergoes expansion according to the following graph. Calculate ...
Text Solution
|