Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
JEE MAINS PREVIOUS YEAR-JEE MAIN-All Questions
- An asteroid of mass m (m lt lt mE) is approaching with a velocity 12 k...
Text Solution
|
- In H–spectrum wavelength of 1^(st) line of Balmer series is lambda= 65...
Text Solution
|
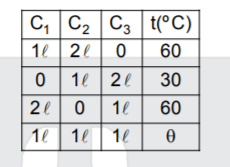
- There are three containers C1, C2 and C3 filled with same material at ...
Text Solution
|
- Two batteries (connected in series) of same emf 10 V of internal resis...
Text Solution
|
- An EMW is travelling along z-axis vecB=5 xx 10^(-8) hatj T, C=3 xx 10^...
Text Solution
|
- Kinetic energy of the particle is E and it's De-Broglie wavelength is ...
Text Solution
|
- The dimensional formula of sqrt((hc^5)/G) is
Text Solution
|
- Two immiscible liquids of refractive index sqrt 2 and 2sqrt2 are fille...
Text Solution
|
- The identical solid sphere each having mass m and diameter d are touch...
Text Solution
|
- A solid sphere having radius R and uniform charge density rho has a ca...
Text Solution
|
- Consider an infinitely long current carrying cylindrical straight wire...
Text Solution
|
- Find current in the wire BC.
Text Solution
|
- Two electromagnetic waves are moving in free space whose electric fiel...
Text Solution
|
- Two ideal di-atomic gases A and B. A is rigid, B has an extra degree o...
Text Solution
|
- An ideal liquid (water) flowing through a tube of non-uniform cross se...
Text Solution
|
- A screw gauge advances by 3mm in 6 rotations. There are 50 divisions o...
Text Solution
|
- A telescope of aperture diameter 5m is used to observe the moon from t...
Text Solution
|
- A particle of mass m is revolving around a planet in a circular orbit ...
Text Solution
|
- Two particles of same mass 'm' moving with velocities vecv1= vhati, an...
Text Solution
|
- Three waves of same intensity (I0) having initial phases 0, pi/4 , - ...
Text Solution
|