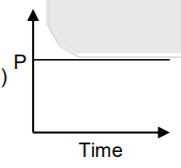
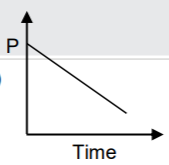
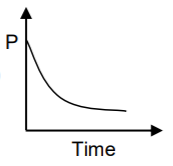
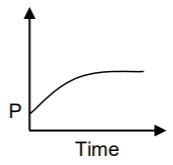
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In a box a mixture containing H2, O2 and CO along with charcoal is pre...
Text Solution
|
- In a box a mixture containing H2, O2 and CO along with charcoal is pre...
Text Solution
|
- 1 mole of sample of O2 and 3 mole sample of H2 are mixed isothermally ...
Text Solution
|
- A mixture of one mole of each of O2(g), H2(g) , He(g) exists in a cont...
Text Solution
|
- Calculate the partial pressures of O2 and H2 in a mixture of 3 moles o...
Text Solution
|
- Calculate the partial pressures of O2 and H2 in a mixture of 3 moles o...
Text Solution
|
- Calculate the partial pressures of O2 and H2 in a mixture of 3 moles o...
Text Solution
|
- Calculate the partial pressures of O2 and H2 in a mixture of 3 moles o...
Text Solution
|
- Calculate the partial pressures of O2 and H2 in a mixture of 3 moles o...
Text Solution
|