Similar Questions
Explore conceptually related problems
Recommended Questions
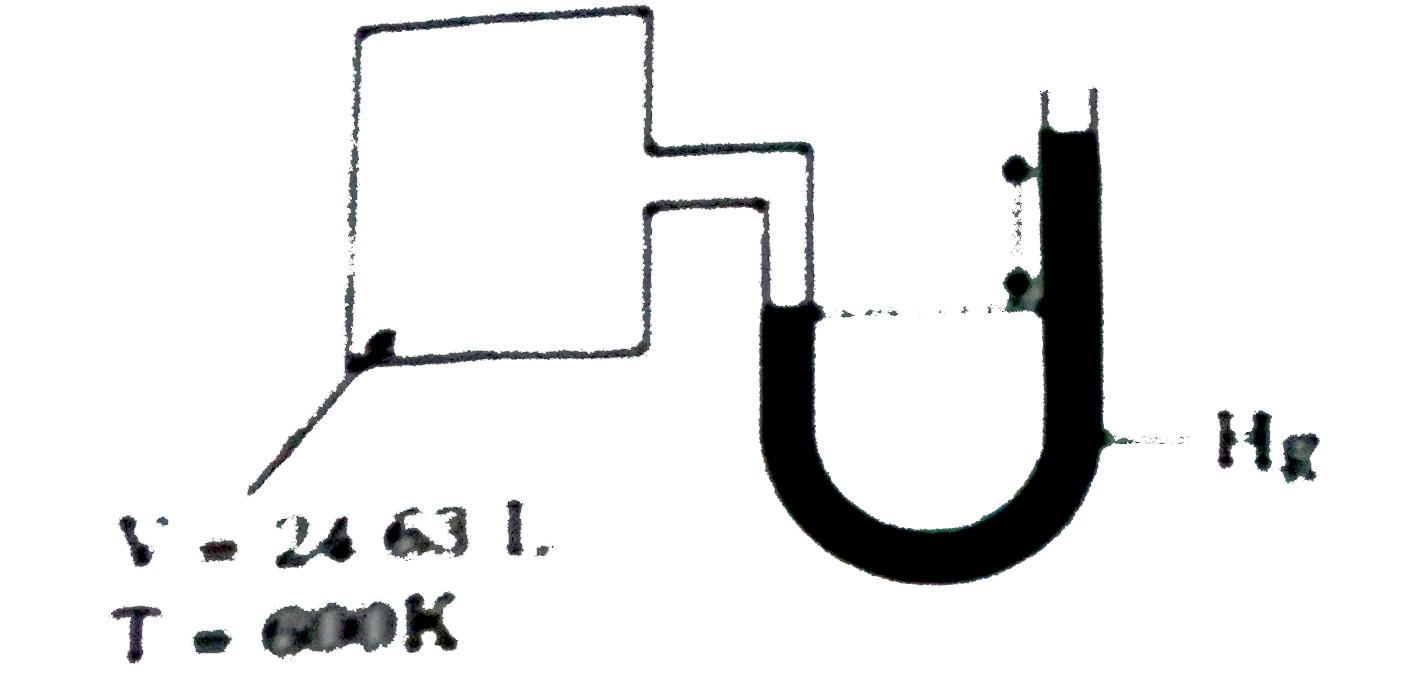
- From the following set-up, calculate moles of the gas present in the c...
Text Solution
|
- In a constant volume gas thermometer, the pressure of the working gas ...
Text Solution
|
- From the following set-up, calculate moles of the gas present in the c...
Text Solution
|
- A manometer reads the pressure of a gas in an enclosure as shown in th...
Text Solution
|
- Arrange the following steps in proper sequence to determine the pressu...
Text Solution
|
- A manometer is connected to gas container. Then the mercury level rise...
Text Solution
|
- A gas is filled into a bulb connected to an open limb manometer. The l...
Text Solution
|
- किसी मैनोमीटर में यदि गैस का दाब, वायुमण्डलीय दाब के बराबर हो तो नालिय...
Text Solution
|
- An open-end manometer was used to determine the pressure of a gas pres...
Text Solution
|