Similar Questions
Explore conceptually related problems
Recommended Questions
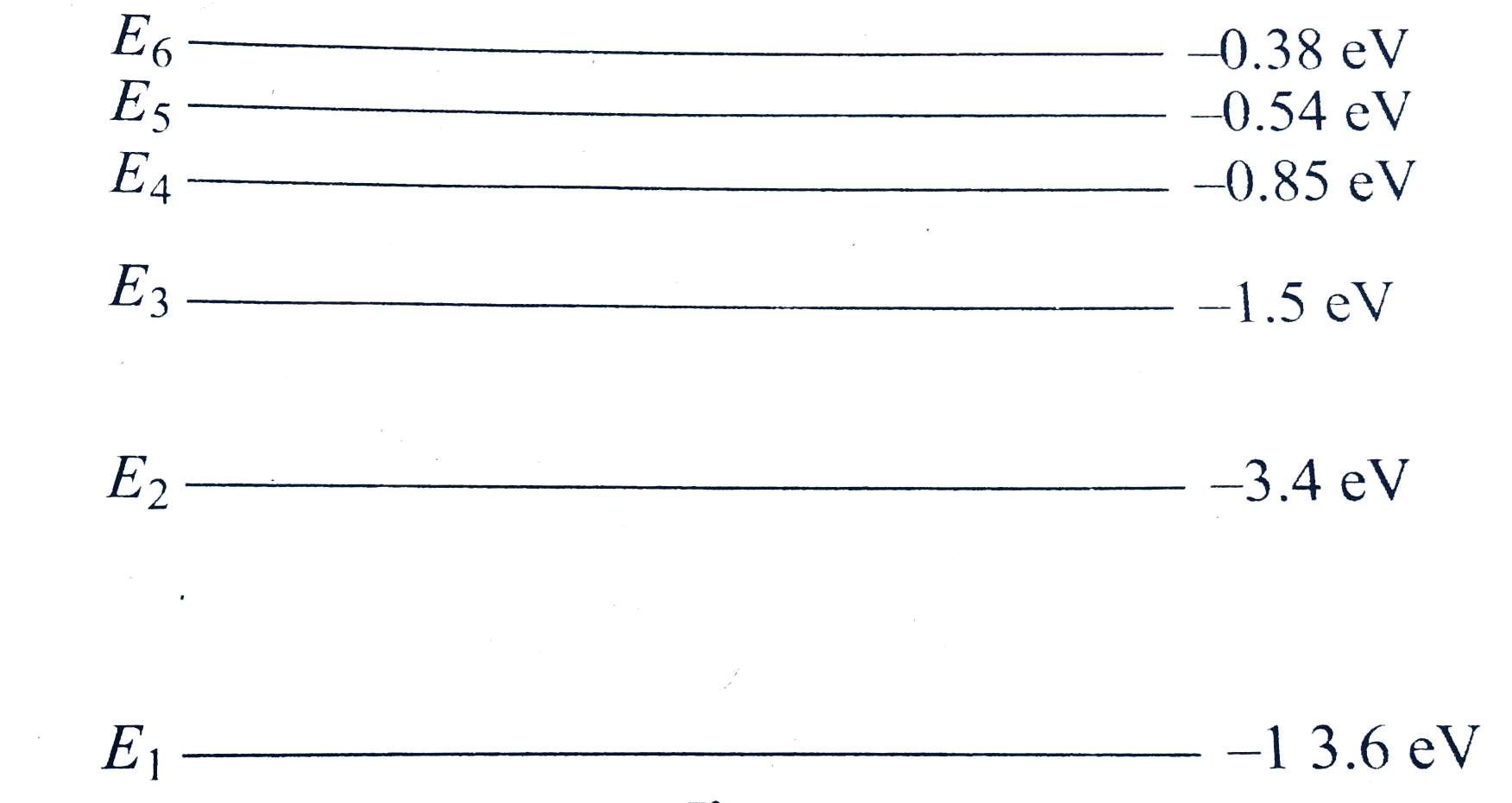
- In figure E(1) and E(2) represent some of the energy levels of the hyd...
Text Solution
|
- The energy levels of an atom are as shown in figure . Which one of tho...
Text Solution
|
- Figure represent some of the lower energy level of the hydrogen atom i...
Text Solution
|
- In figure E(1) and E(2) represent some of the energy levels of the hyd...
Text Solution
|
- An atom emits a spectral line of wavelength lambda when an electron ma...
Text Solution
|
- An electron in hydrogen atom is in the n=4 energy level. When it makes...
Text Solution
|
- In the ultraviolet region of the atomic spectrum of hydrogen, a line i...
Text Solution
|
- एक हाइड्रोजन परमाणु में कक्षा n = 3 से कक्षा n = 2 में एक संक्रमण होता...
Text Solution
|
- Which of the following transition in a hydrogen atom produces a photon...
Text Solution
|