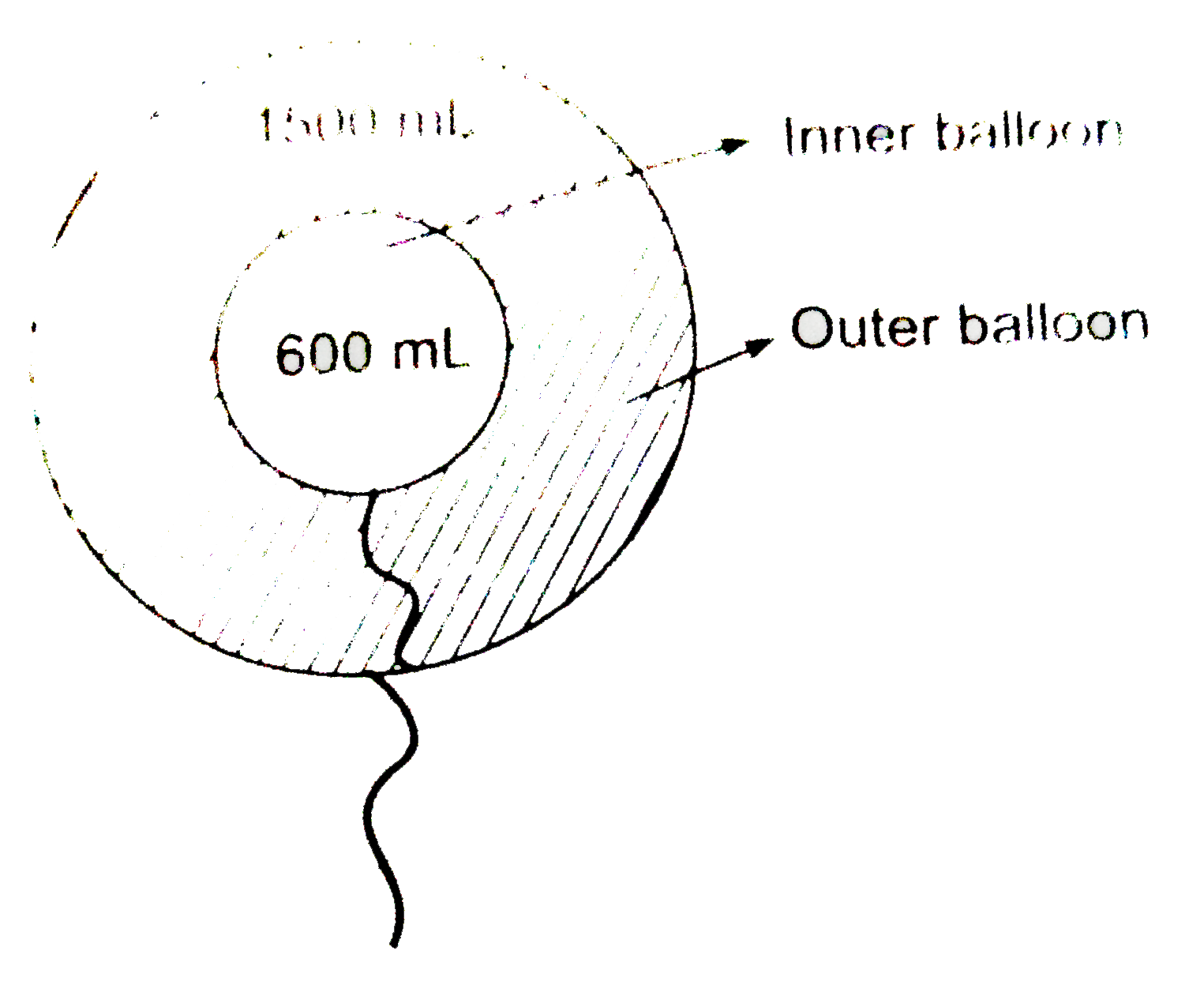Similar Questions
Explore conceptually related problems
Recommended Questions
- Two inflated ballons I and II (thin skin) having volume 600 mL and 150...
Text Solution
|
- A bolloon blown up has a volume of 300 mL at 27% C. The balloon is dis...
Text Solution
|
- Two inflated ballons I and II (thin skin) having volume 600 mL and 150...
Text Solution
|
- Two balloon A and B are taken at 300 K. Maximum capacity of balloon A ...
Text Solution
|
- Which of the following respiratory volume has the volume of 1100 mL to...
Text Solution
|
- A toy balloon blown up at 5^(@)C has a volume of 480 mL. At this stage...
Text Solution
|
- Two inflated ballons I and II (thin skin) having volume 600 mL and 150...
Text Solution
|
- At 740 torr pressure volume of N(2) gas is 800 mL, if volume becomes 5...
Text Solution
|
- A blown-up balloon has a volume of 500 mL at 5^@C . The balloon is dis...
Text Solution
|