Similar Questions
Explore conceptually related problems
Recommended Questions
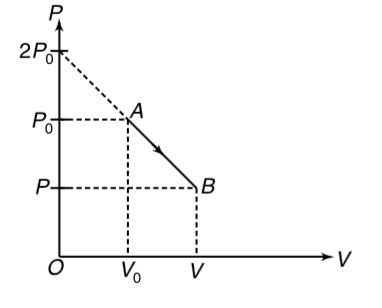
- One mole of an ideal gas is expanded from the state A(P(0), V(0)) to f...
Text Solution
|
- One mole of an ideal gas at pressure P(0) and temperature T(0) is expa...
Text Solution
|
- One mole of an ideal gas goes from an initial state A to final state B...
Text Solution
|
- A gas is found to obey the law P^(2)V = constant. The initial temperat...
Text Solution
|
- An ideal monatomic gas is at P(0), V(0). It is taken to final volume 2...
Text Solution
|
- n moles of an ideal mono atomic gas is initially at pressure 32 P(0) a...
Text Solution
|
- One mole of an ideal gas is expanded from the state A(P(0), V(0)) to f...
Text Solution
|
- One mole of an ideal gas undergoes a process P=P(0)-alphaV^(2) whe...
Text Solution
|
- One mole of an ideal gas goes from an initial state A to final state B...
Text Solution
|