Similar Questions
Explore conceptually related problems
Recommended Questions
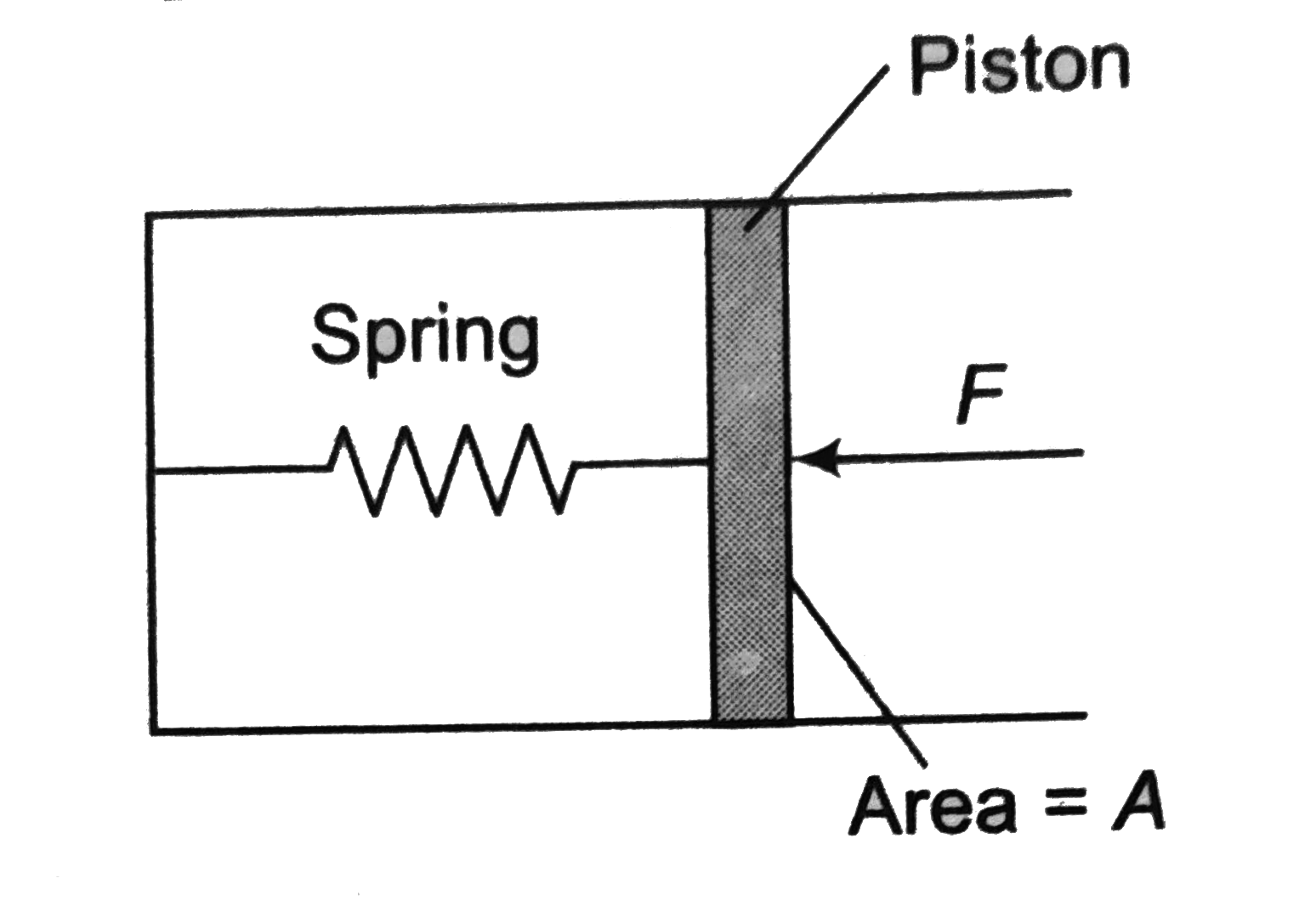
- The pressure gauge shown in figure has a spring for which k=60N//m and...
Text Solution
|
- A cylidrical vesel containing a liquid is closed by a smooth piston o...
Text Solution
|
- The pressure gauge shown in figure has a spring for which k=60N//m and...
Text Solution
|
- A cylinder is fitted with a piston, beneath which is a spring, as in t...
Text Solution
|
- In the arrangement shown in Fig. gas is thermally insulated. An ideal ...
Text Solution
|
- A fixed container is fitted with a piston which is attached to a sprin...
Text Solution
|
- A smooth air-tight piston connected to a spring of force constant k an...
Text Solution
|
- A manometer reads the pressure of a gas in an enclosure as shown in th...
Text Solution
|
- If the gauge pressure, at the bottom of a water tank is 2.7 kPa, what ...
Text Solution
|