Similar Questions
Explore conceptually related problems
Recommended Questions
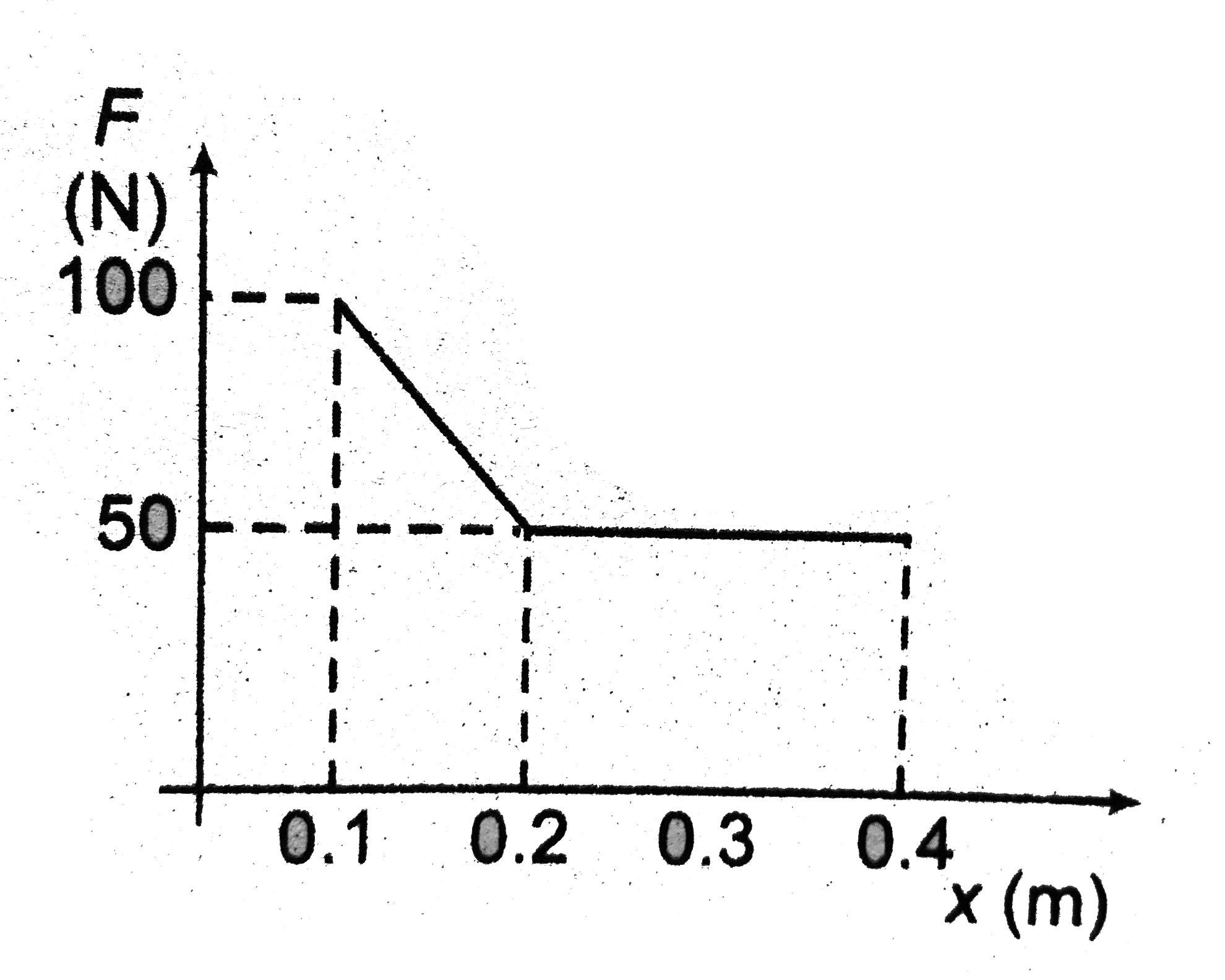
- The given figure shows the variation of force applied by ideal gas on ...
Text Solution
|
- The given figure shows the variation of force applied by ideal gas on ...
Text Solution
|
- In a process, 701 J of heat is absorbed by a system and 394 J of work ...
Text Solution
|
- A system undergoes a process which absorbed 5 KJ of heat and undergoin...
Text Solution
|
- The given figrue shows the variation of force exerted on the piston by...
Text Solution
|
- In a process 701 J of heat is absorbed by a system and 394 J of work i...
Text Solution
|
- In a process, 701 J of heat is absorbed by a system and 394 J of work ...
Text Solution
|
- In a process, 701 J of heat is absorbed by a system and 394 J of work ...
Text Solution
|
- Figure Shows a process ABCA performed on an ideal gas. Find the net he...
Text Solution
|