Similar Questions
Explore conceptually related problems
Recommended Questions
- 100 moles of an ideal monatomic gas undergoes the thermodynamic proces...
Text Solution
|
- 100 moles of an ideal monatomic gas undergoes the thermodynamic proces...
Text Solution
|
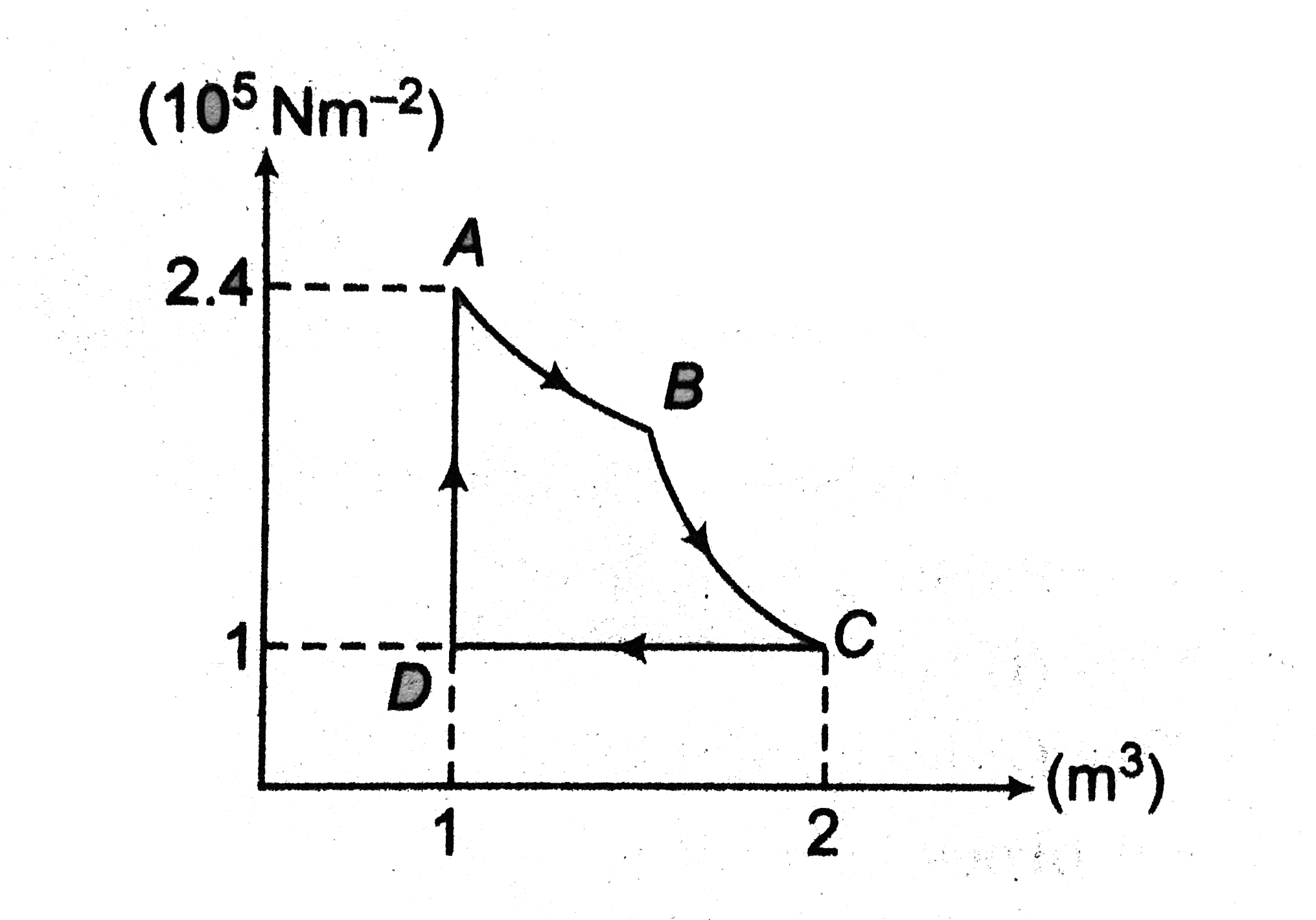
- One mole of an ideal gas is taken through the cyclic process ABCDA, as...
Text Solution
|
- One mole of an ideal gas is carried through a thermodynamics cycle as ...
Text Solution
|
- Two moles of a monoatomic ideal gas undergoes a process AB as shown in...
Text Solution
|
- In an adiabatic process, the work involed during expansion or compr...
Text Solution
|
- In adiabatic process, the work involved during expansion or compressio...
Text Solution
|
- An ideal gas undergoes a cyclic process as shown. Part of the process ...
Text Solution
|
- संलग्न चित्र में, एक आदर्श गैस की ऊष्मागतिकीय प्रक्रियाओं का P-V। ग्रा...
Text Solution
|