Similar Questions
Explore conceptually related problems
Recommended Questions
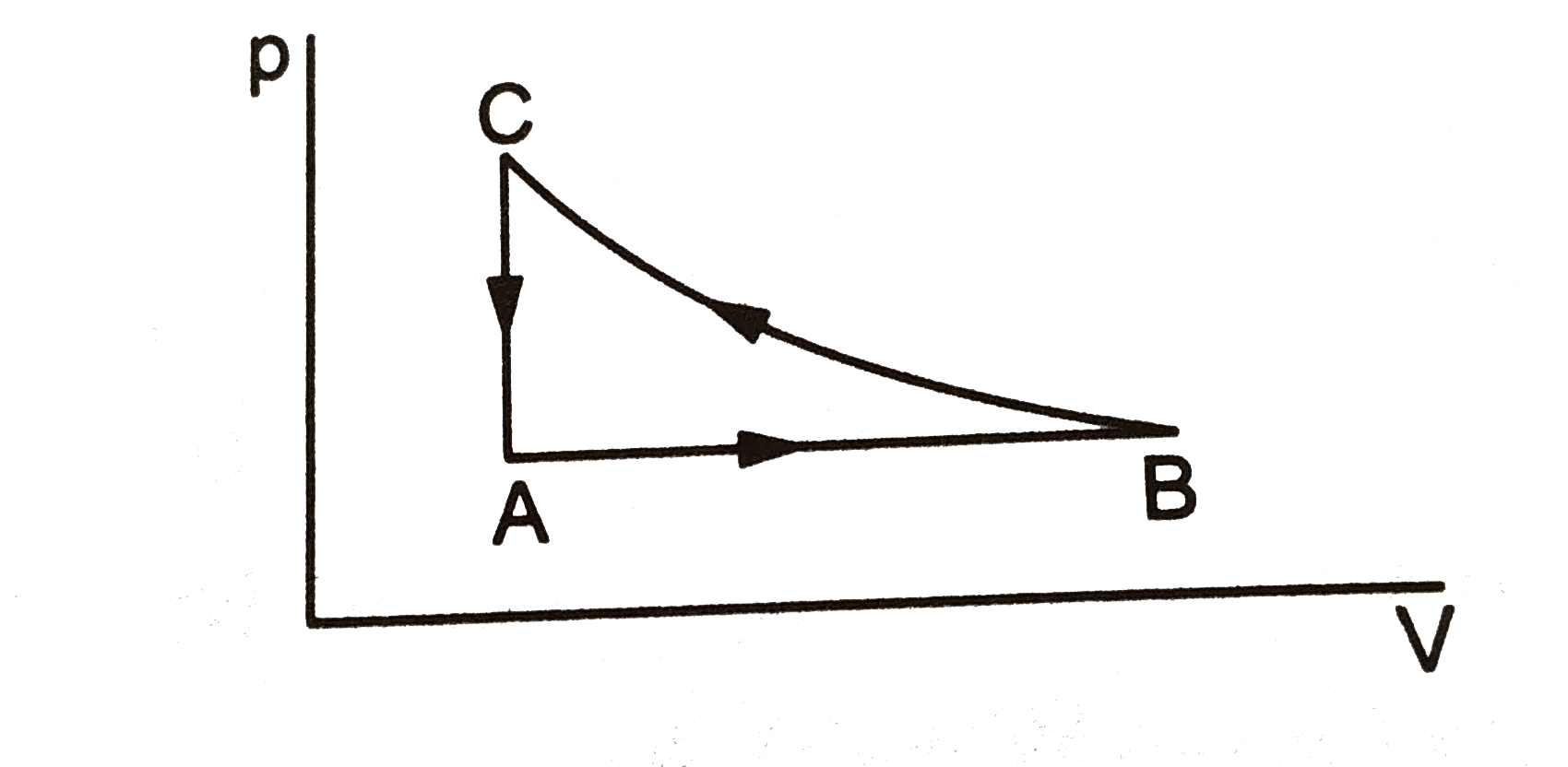
- consider the cyclic process ABCA on a sample of 2.0 mol of an ideal ga...
Text Solution
|
- consider the cyclic process ABCA on a sample of 2.0 mol of an ideal ga...
Text Solution
|
- Consider the cyclic process ABCA, shown in, performed on a sample of ...
Text Solution
|
- In figure, a cyclic process ABCA of 3 moles of an ideal gas is given. ...
Text Solution
|
- In figure, a sample of 3 moles of an ideal gas is undergoing through a...
Text Solution
|
- Consider the cyclic process abca performed on a sample of 2.0 mole of ...
Text Solution
|
- A sample of ideal gas is taken through the cyclic process shown in the...
Text Solution
|
- consider the cyclic process ABCA on a sample of 2.0 mol of an ideal ga...
Text Solution
|
- Consider the cyclic process ABCA, shown in fig, performed on a sample ...
Text Solution
|