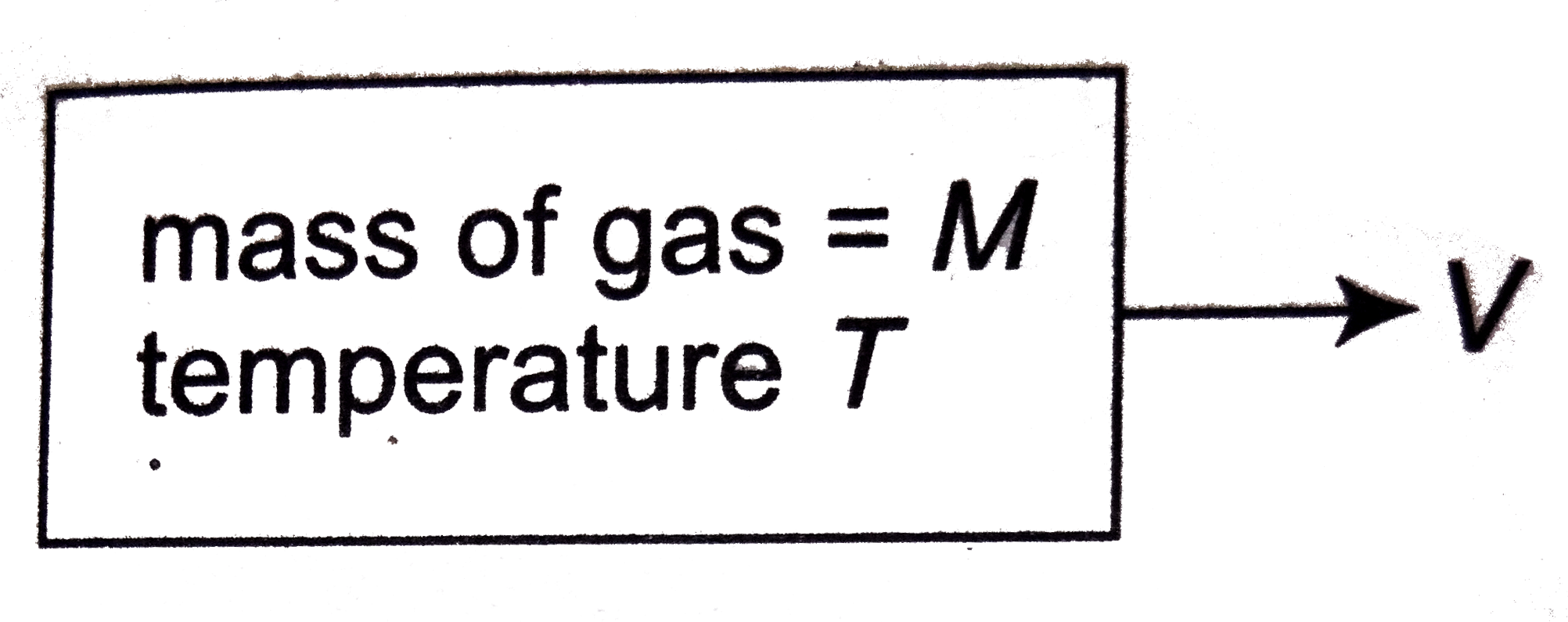Similar Questions
Explore conceptually related problems
Recommended Questions
- A light container having a diatomic gas enclosed with in is moving wit...
Text Solution
|
- A light container having a diatomic gas enclosed with in is moving wit...
Text Solution
|
- A light container having a diatomic gas enclosed with in is moving wit...
Text Solution
|
- A light container having a diatomic gas enclosed within is moving with...
Text Solution
|
- A thermally insulated vessel containing diatomic gas of molar mass M i...
Text Solution
|
- If the total kinetic energy per unit volume of gas enclosed in a conta...
Text Solution
|
- गतिज गैस समीकरण के आधार पर किसी गैस के मोल की कुल गतिज ऊर्जा तथा किसी ...
Text Solution
|
- A light container having a diatomic gas enclosed with in is moving wit...
Text Solution
|
- n moles of diatomic gas in a cylinder is at a temperature T . Heat is ...
Text Solution
|