Similar Questions
Explore conceptually related problems
Recommended Questions
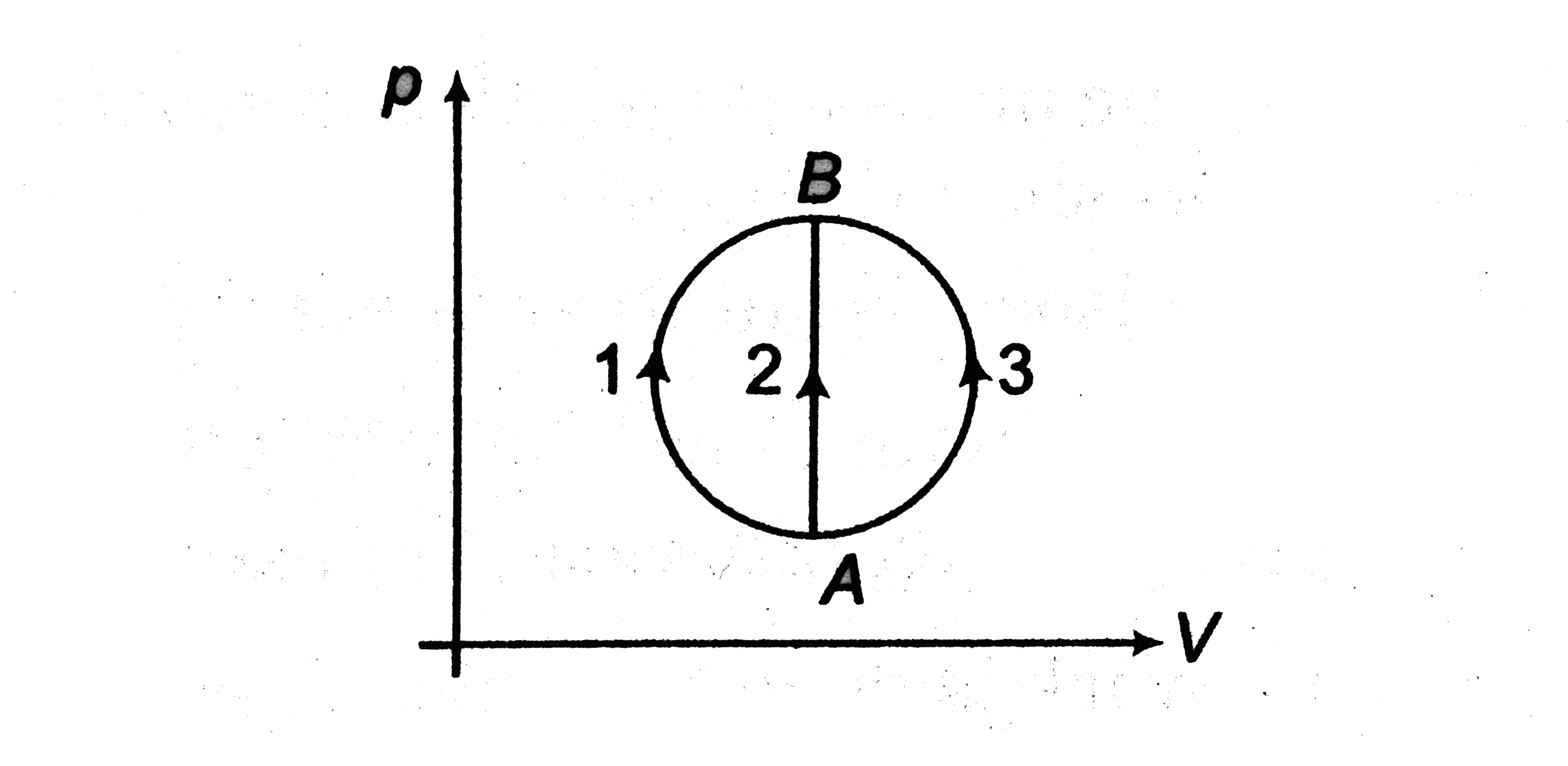
- A gas undergoes A to B through three different processes 1,2 and 3 as ...
Text Solution
|
- Let Vr denote the sum of the first r terms of an arithmetic progressio...
Text Solution
|
- Three charges -q1 , +q2 and -q3 are placed as shown in the figure. The...
Text Solution
|
- A gas undergoes A to B through three different processes 1,2 and 3 as ...
Text Solution
|
- Temperature of a monoatomic gas is increased from T0 to 2T0 in three d...
Text Solution
|
- For an ideal gas four processes are marked as 1,2,3 and 4 on P-V diagr...
Text Solution
|
- graph of an ideal gas is as shown in figure. Heat is supplied to the g...
Text Solution
|
- एक द्विपरमाण्विक गैस के स्थिर दाब तथा स्थिर आयतन पर एकांक ताप परिवर्तन...
Text Solution
|
- The value of charge q1 ,q2 and q3 as shown in the figure are
Text Solution
|