Similar Questions
Explore conceptually related problems
Recommended Questions
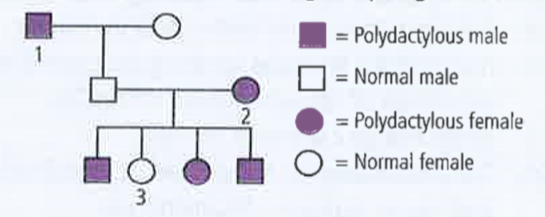
- In humans polydactyly (i.e., presence of extra fingers and toes) is de...
Text Solution
|
- In humans polydactyly (i.e., presence of extra fingers and toes) is de...
Text Solution
|
- Fused ear lobes appear in the progeny due to an autosomal recessive ge...
Text Solution
|
- In the pedigree given below, indicate whether the shaded symbols indic...
Text Solution
|
- In the given pedigree, indicate whether the shaded symbols indicate do...
Text Solution
|
- In the given pedigree, indicate whether the shaded symbols indicate do...
Text Solution
|
- In humans, polydactyly (i.e. presence of extra fingers and toes) is de...
Text Solution
|
- Differentiate dominant allele and recessive allele.
Text Solution
|
- If the frequency of occurrence of dominant allele A id p and that of r...
Text Solution
|