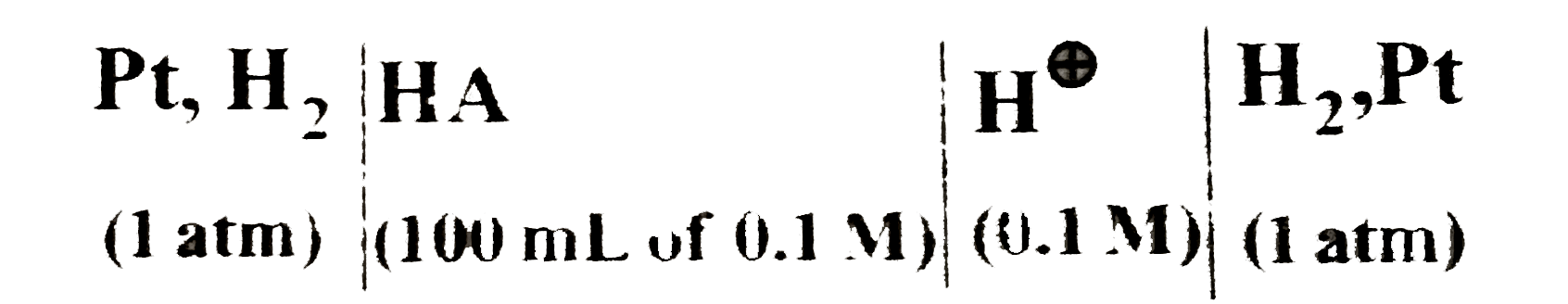Similar Questions
Explore conceptually related problems
Recommended Questions
- The EMF of the following cell is observed to be 0.118V at 25^(@)C: ...
Text Solution
|
- The emf of the following cell is observed to be 0.118V at 25^(@)C . [P...
Text Solution
|
- The EMF of the following cell is 0.180 V at 30^(@)C. Find EMF(ce...
Text Solution
|
- The EMF of the following cell is observed to be 0.118V at 25^(@)C: If ...
Text Solution
|
- A cell contains two hydrogen electrodes. The negative electrode is in ...
Text Solution
|
- The emf of the cell, Pt|H(2)(1atm)|H^(+) (0.1M, 30mL)||Ag^(+) (0.8M)Ag...
Text Solution
|
- A reversible galvanic cell is connected to an external battery. If the...
Text Solution
|
- Assertion: Terminal voltage of a cell is greater than emf of cell duri...
Text Solution
|
- A cell of emf 10 V and internal resistance 3 Omega is connected in par...
Text Solution
|