Similar Questions
Explore conceptually related problems
Recommended Questions
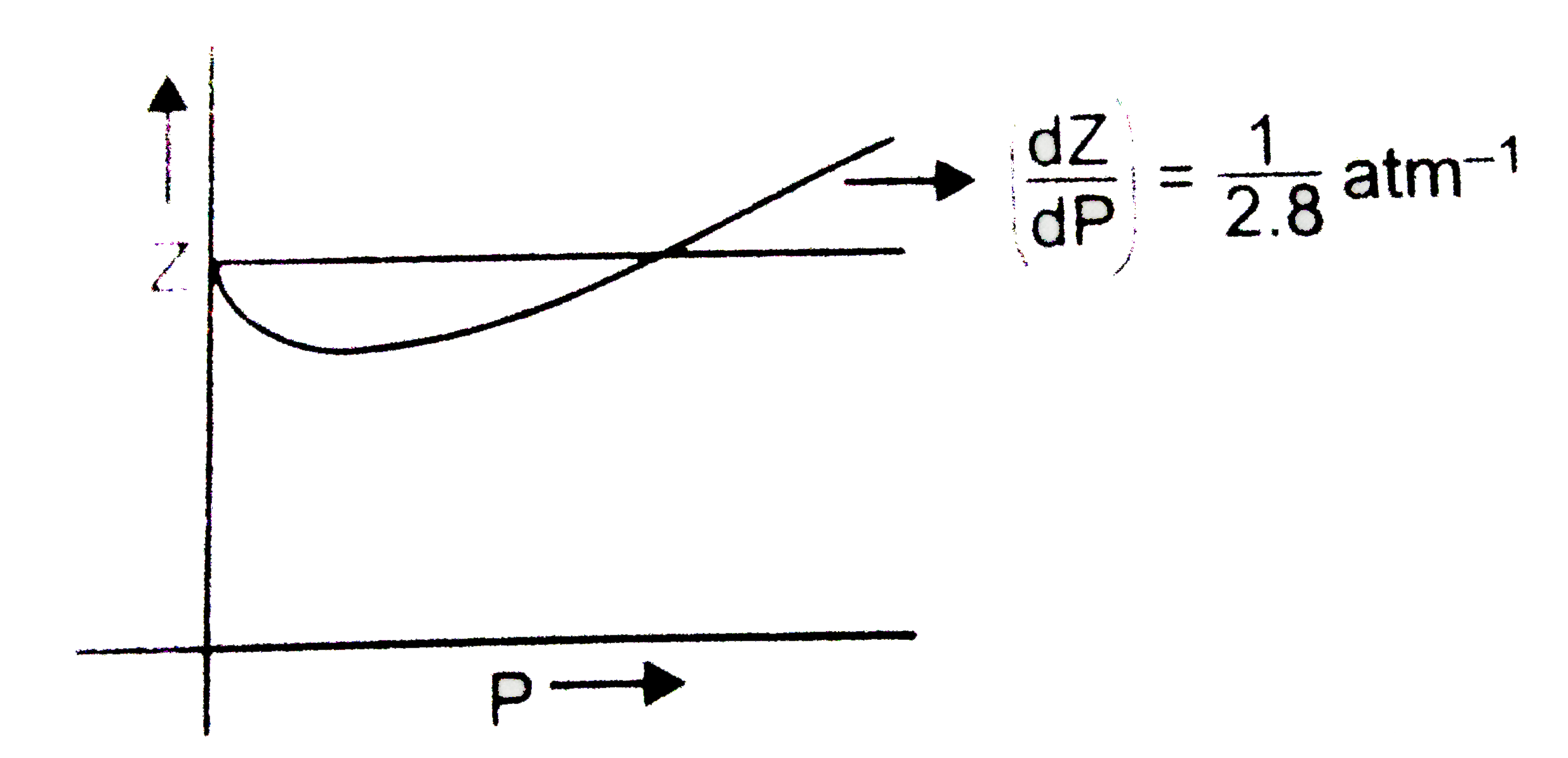
- The graph of compressibility factor (Z) vs. P for one mole of a real g...
Text Solution
|
- For 1 mole of an ideal gas, a graph of pressure vs volume is plotted a...
Text Solution
|
- For 1 mole of an ideal gas, a graph of pressure vs volume is plotted a...
Text Solution
|
- P vs V graph is plotted for 1 mole of hypothetical gas. Range of a/b f...
Text Solution
|
- Consider the given graph: Graph is plotted for 1 mol of gas at 400K, f...
Text Solution
|
- Z us P graph is plotted for 1 mole of hypothetical gas. Volume of gas ...
Text Solution
|
- A graph is plotted between p (atm) vs t^(@)C for 10 mol of an ideal ga...
Text Solution
|
- The graph of compressibility factor (Z) vs. P for one mole of a real g...
Text Solution
|
- The volume vs. temperature graph of 1 mole of an ideal gas is given be...
Text Solution
|