Similar Questions
Explore conceptually related problems
Recommended Questions
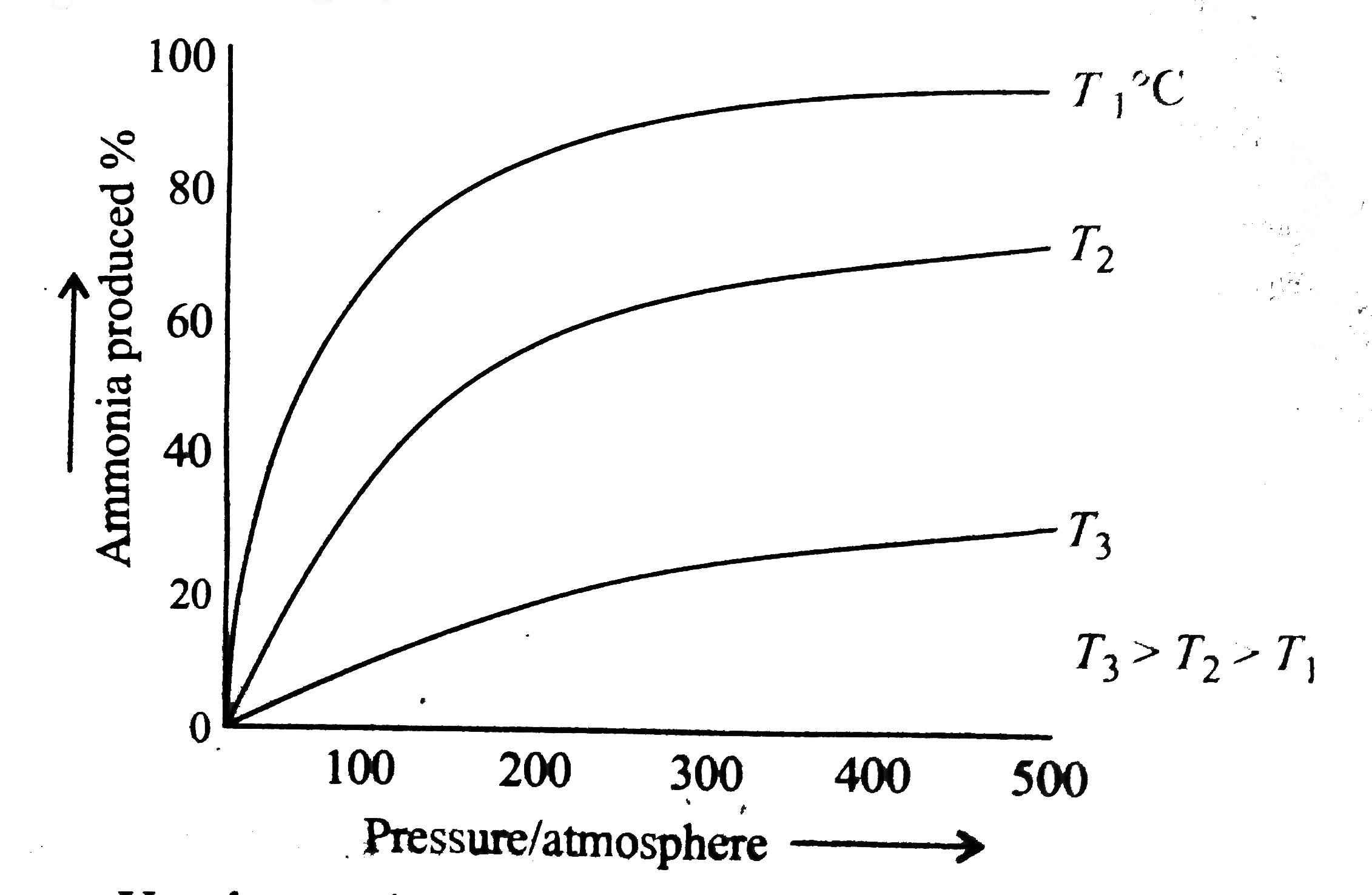
- The persentage of ammonia produced from nitrogen and hydrogen under co...
Text Solution
|
- The persentage of ammonia produced from nitrogen and hydrogen under co...
Text Solution
|
- The persentage of ammonia produced from nitrogen and hydrogen under co...
Text Solution
|
- The persentage of ammonia produced from nitrogen and hydrogen under co...
Text Solution
|
- A mixture of nitrogen and hydrogen in the ratio of 1: 3 reach equilibr...
Text Solution
|
- Nitrogen and hydrogen combine to form ammonia under high temperature a...
Text Solution
|
- Ammonia dissociates to give nitrogen and hydrogen . What happens if th...
Text Solution
|
- नाइट्रोजन एवं हाइड्रोजन उच्च ताप एवं दाब की स्थितियों में संयुक्त होकर...
Text Solution
|
- কোন শর্তে নাইট্রোজেন ও হাইড্রোজেন বিক্রিয়া করে অ্যামোনিয়া উৎপণ্ন করে?...
Text Solution
|