A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
KINETIC THEORY OF GASES
HC VERMA|Exercise Objective 2|7 VideosKINETIC THEORY OF GASES
HC VERMA|Exercise Exercises|62 VideosKINETIC THEORY OF GASES
HC VERMA|Exercise Short Answer|14 VideosINTRODUCTION TO PHYSICS
HC VERMA|Exercise Exercises|19 VideosLAWS OF THERMODYNAMICS
HC VERMA|Exercise Short Answer|15 Videos
Similar Questions
Explore conceptually related problems
HC VERMA-KINETIC THEORY OF GASES-Objective 1
- Which of the following parameters is the same for molecules of all gas...
Text Solution
|
- A gas behaves more closely as an ideal gas at
Text Solution
|
- The pressure of an ideal gas is weitten as p=(2E)/(3V).Here E refers t...
Text Solution
|
- The energy of a given sample of an ideal gas depends only on its
Text Solution
|
- Which of the following gases has maximum rms speed at a given tempera...
Text Solution
|
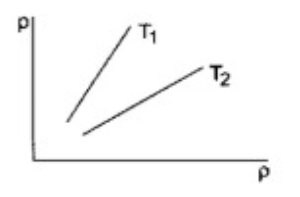
- Shows graphs of pressure vs density for an ideal gas at two temperatur...
Text Solution
|
- The mean square speed of the molecules of a gas at absolute temoeratur...
Text Solution
|
- Suppose a container is evacuated to leave just one molecule of a gas i...
Text Solution
|
- The rms speed of oxygen at room temperature is about 500m/s.The rms sp...
Text Solution
|
- The pressure of a gas kept in an isothermal container is 200 kPa. If h...
Text Solution
|
- The rms speed of pzygen molecules in a gas in a gas is v. If the tempe...
Text Solution
|
- The quantity(pV)/(kt) represents
Text Solution
|
- The process on an ideal gas, shown in figure,is
Text Solution
|
- There is some liquid in a closed bottle. The amount of liquid is conti...
Text Solution
|
- There is some liquid in a closed bottle. The amount of liquid remains ...
Text Solution
|
- Vapour is injected at a uniform rate in a closed vessel which was init...
Text Solution
|
- A vessel A has volume V and a vessel B has volume 2V. Both contain som...
Text Solution
|