Similar Questions
Explore conceptually related problems
Recommended Questions
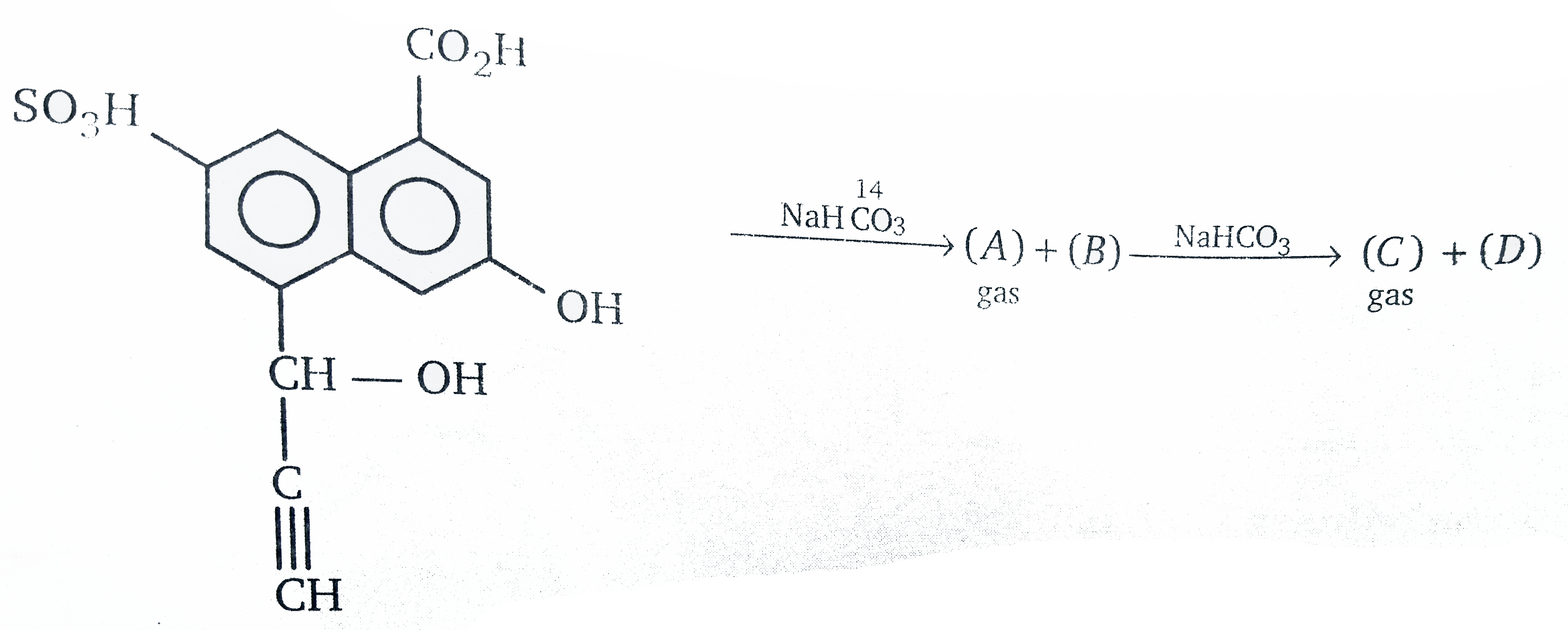
- Sum of molecular mass of gas (A+C) is :
Text Solution
|
- Sum of molecular mass of gas (A+C) is :
Text Solution
|
- The density of a gas A is three times that of a gas B. It the molecula...
Text Solution
|
- Molecular mass of a gas is 78 . Its density at 98 ^(@)C and 1 atm will...
Text Solution
|
- The density of a gas A is three times that of a gas B. It the molecula...
Text Solution
|
- The density of a gas 'A' is three timess that of a gas 'B' if the mole...
Text Solution
|
- Molecular mass is the sum of .
Text Solution
|
- Relative molecular mass of a gas .
Text Solution
|
- At NTP, the mass of 1 litre of gas is 3 g. Molecular mass of the gas i...
Text Solution
|