Similar Questions
Explore conceptually related problems
Recommended Questions
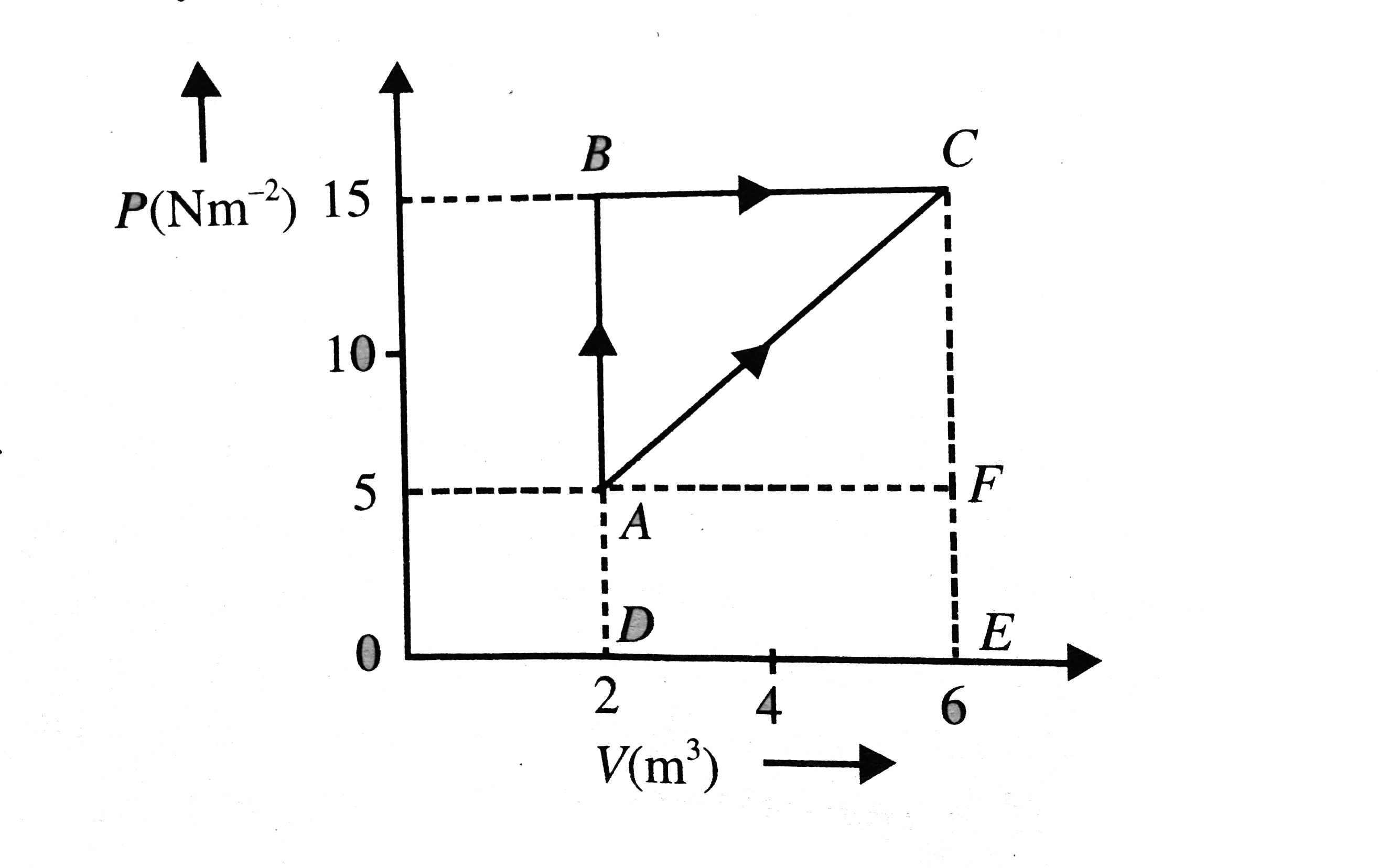
- Figure. Shows an ideal gas changing its state A to state C by two diff...
Text Solution
|
- Figure. Shows an ideal gas changing its state A to state C by two diff...
Text Solution
|
- The given figure shown a change of state A to state C by two paths ABC...
Text Solution
|
- In the figure an ideal gas changes is state from state A to state C by...
Text Solution
|
- In the given figure, an ideal gas changes it state from A to state C b...
Text Solution
|
- In the given figure an ideal gas changes its state from A to state C b...
Text Solution
|
- The given figure shows a change of state A to state C by two paths. AB...
Text Solution
|
- In the given figure, an ideal gas changes its state from state A to st...
Text Solution
|
- In the given figure, an ideal gas changes its state from state A to st...
Text Solution
|