Similar Questions
Explore conceptually related problems
Recommended Questions
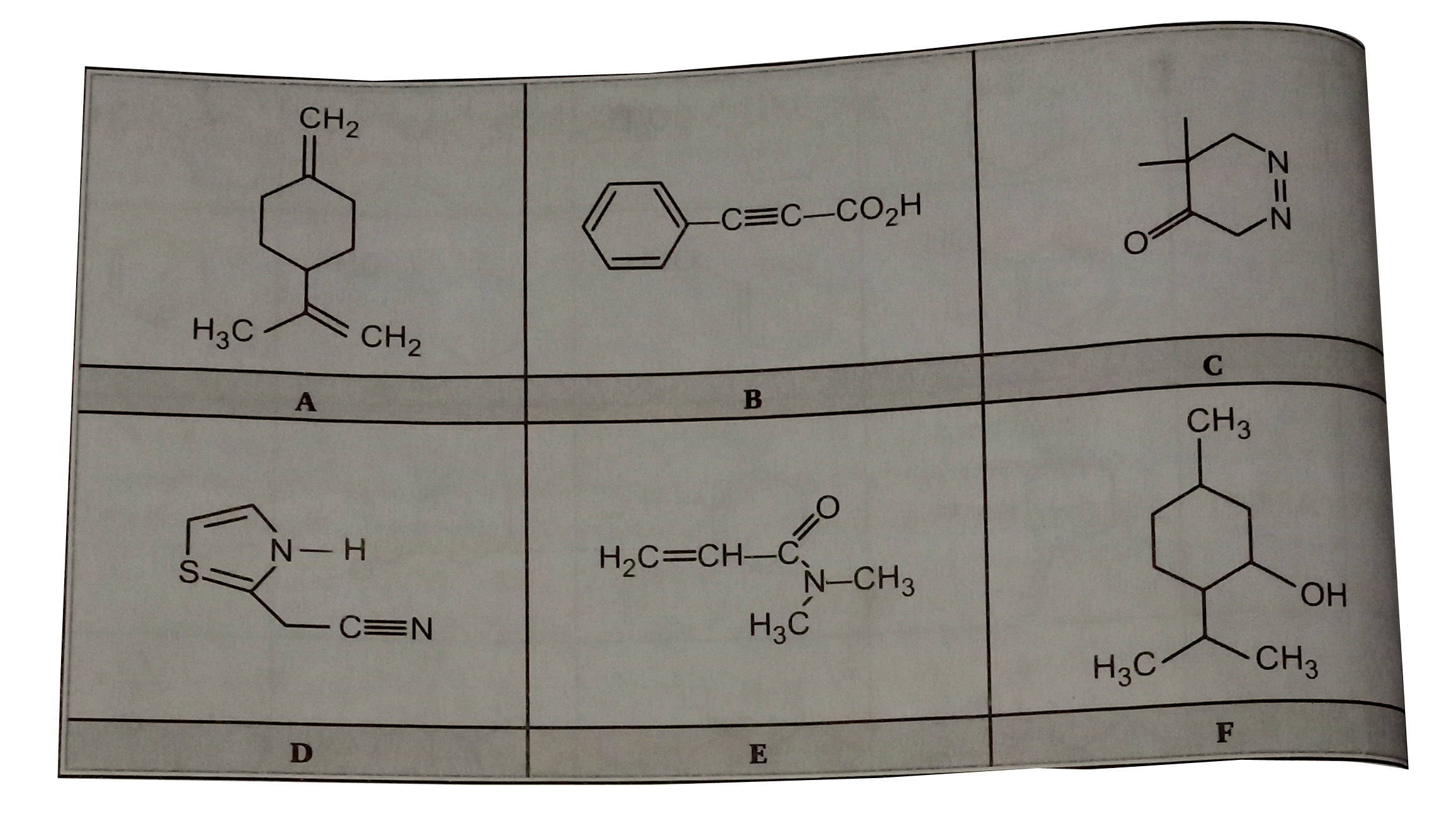
- For each of the six structural formulae (A through F) shown, below, fi...
Text Solution
|
- The following questions refer to the twelve compounds given below. You...
Text Solution
|
- The following questions refer to the twelve compounds given below. You...
Text Solution
|
- The following questions refer to the twelve compounds given below. You...
Text Solution
|
- For each of the six structural formulae (A through F) shown, below, fi...
Text Solution
|
- Ecamine structures a through j, shown below, with reaspect to their sy...
Text Solution
|
- Each of the five questions in a multiple choice test has 4 p...
Text Solution
|
- [" Six different letters are placed in "],[" boxes of the figure as sh...
Text Solution
|
- The following questions refer to the twelve compounds given below. You...
Text Solution
|