A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS-PRACTICE SET 07-PAPER 1( PHYSICS & CHEMISTRY)
- Standard enthalpy of formation is not zero for
Text Solution
|
- Resistance of a conductvity cell filled with a solution of an electrol...
Text Solution
|
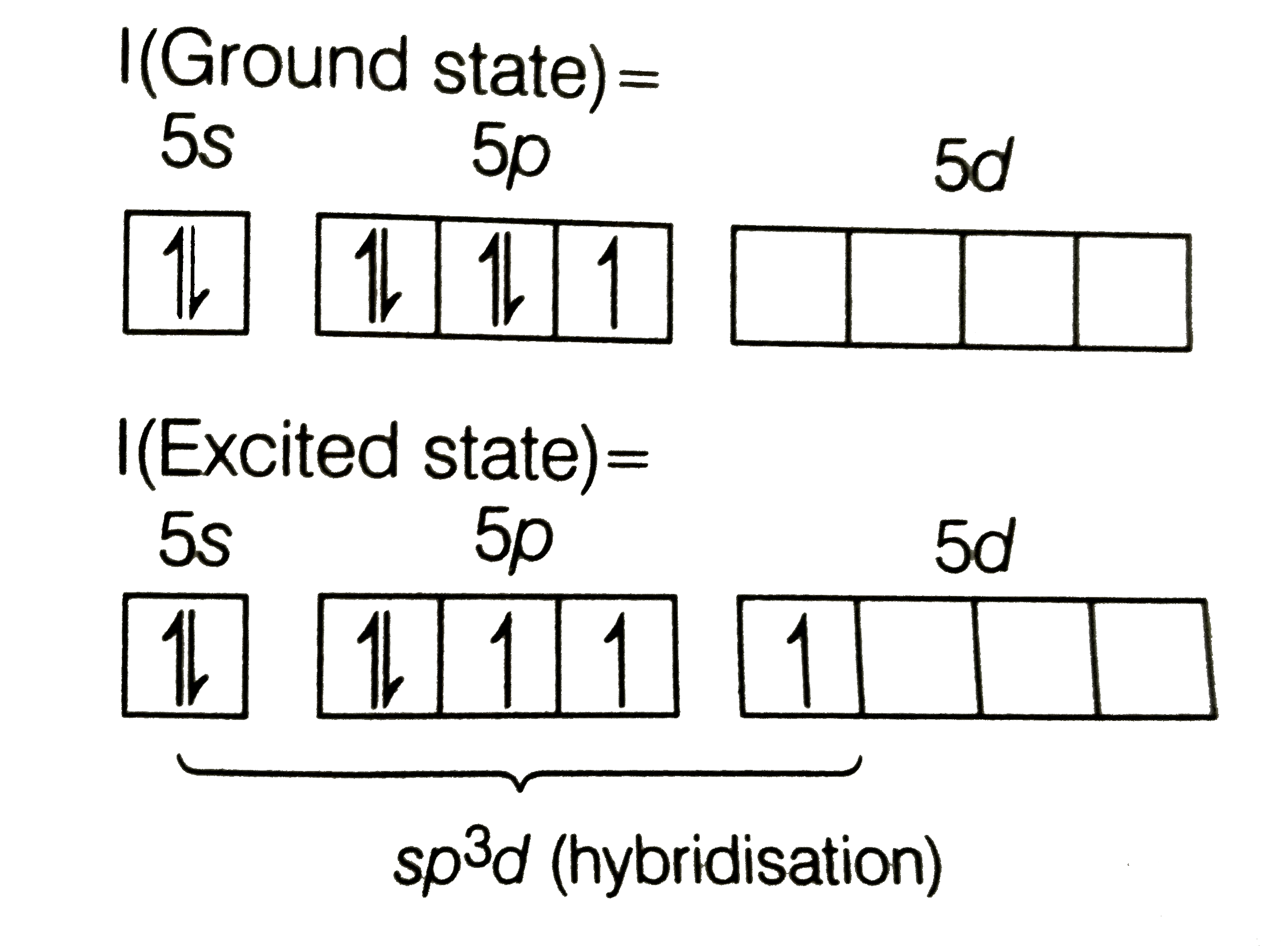
- The hybridisation present in IF(3) is
Text Solution
|
- In the equation of state of an ideal gas PV = nRT, the value of univer...
Text Solution
|
- Oxygen differs from other member of irts family due to
Text Solution
|
- Noble gases are sparingly soluble in water due to
Text Solution
|
- Which of the following reactions can be used for the preparation of 3...
Text Solution
|
- The use of C CI(4) as fire extinguisher depends on the fact that
Text Solution
|
- CH(3)COOH+PCI(5)to A overset(C(6)H(5)) underset("Anhy" AICI(3)) rarrB....
Text Solution
|
- Which one is incorrect about ozone ?
Text Solution
|
- When hydrolysis of AI(4)C(3) occure, which one of the following gas li...
Text Solution
|
- When dimethylglyoxime is added to Ni^(2+), which of the following stat...
Text Solution
|
- Ethanol shows intermolecular H- bonding. Which of the following proper...
Text Solution
|
- When Grignard reagent (CH(3)MgBr) reacts with CO(2), which of the foll...
Text Solution
|
- Ethyl amine on oxidation in the presence of KMnO(4) gives
Text Solution
|
- Reaction of RCN with sodium and alcohol leadd to the formation of
Text Solution
|
- Which of the following statements concering glucose is incorrect ?
Text Solution
|
- Polyacrylonitrile contaisn a linkage of
Text Solution
|
- Buna-S rubber is the polymer of which type ?
Text Solution
|
- Which is the strongest reducing agent?
Text Solution
|