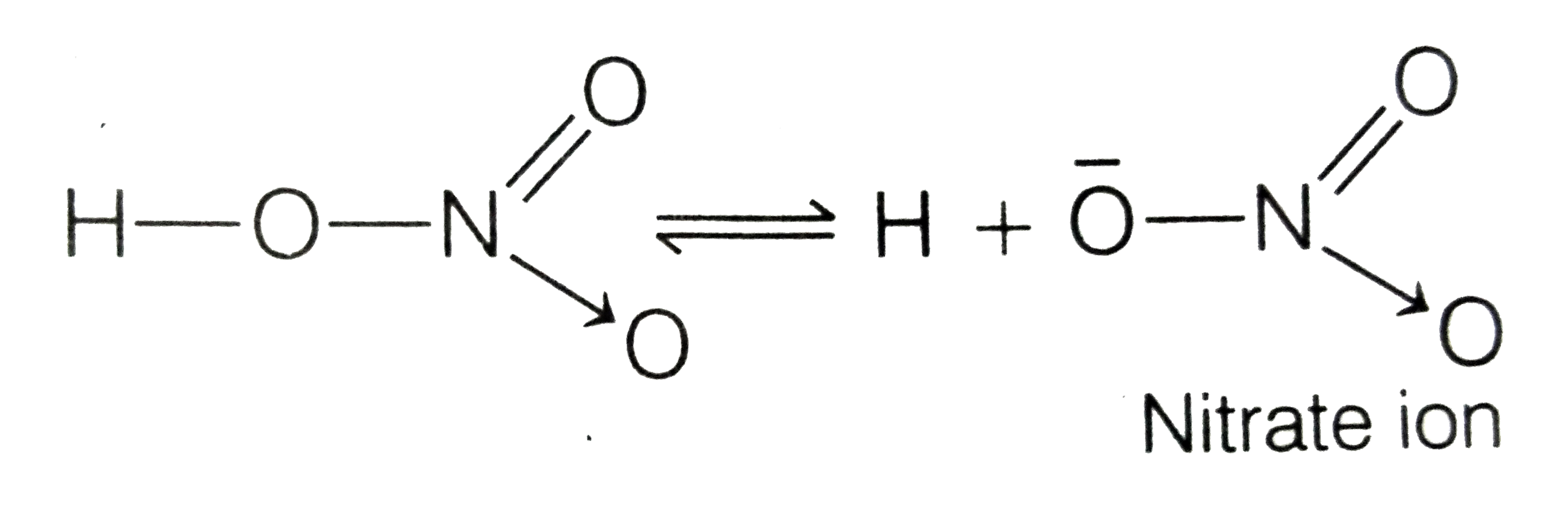
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
PRACTICE SET 08
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS|Exercise PAPER 2 OBJECTIVE TYPE|2 VideosPRACTICE SET 07
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS|Exercise PAPER 1( PHYSICS & CHEMISTRY)|50 VideosPRACTICE SET 09
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS|Exercise PAPER 1 (PHYSICS & CHEMISTRY)|50 Videos
Similar Questions
Explore conceptually related problems