A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS-PRACTICE SET 09-PAPER 1 (PHYSICS & CHEMISTRY)
- Which of the following oxides is not expected to react with sodium hyd...
Text Solution
|
- Wrong about molar conductivity is
Text Solution
|
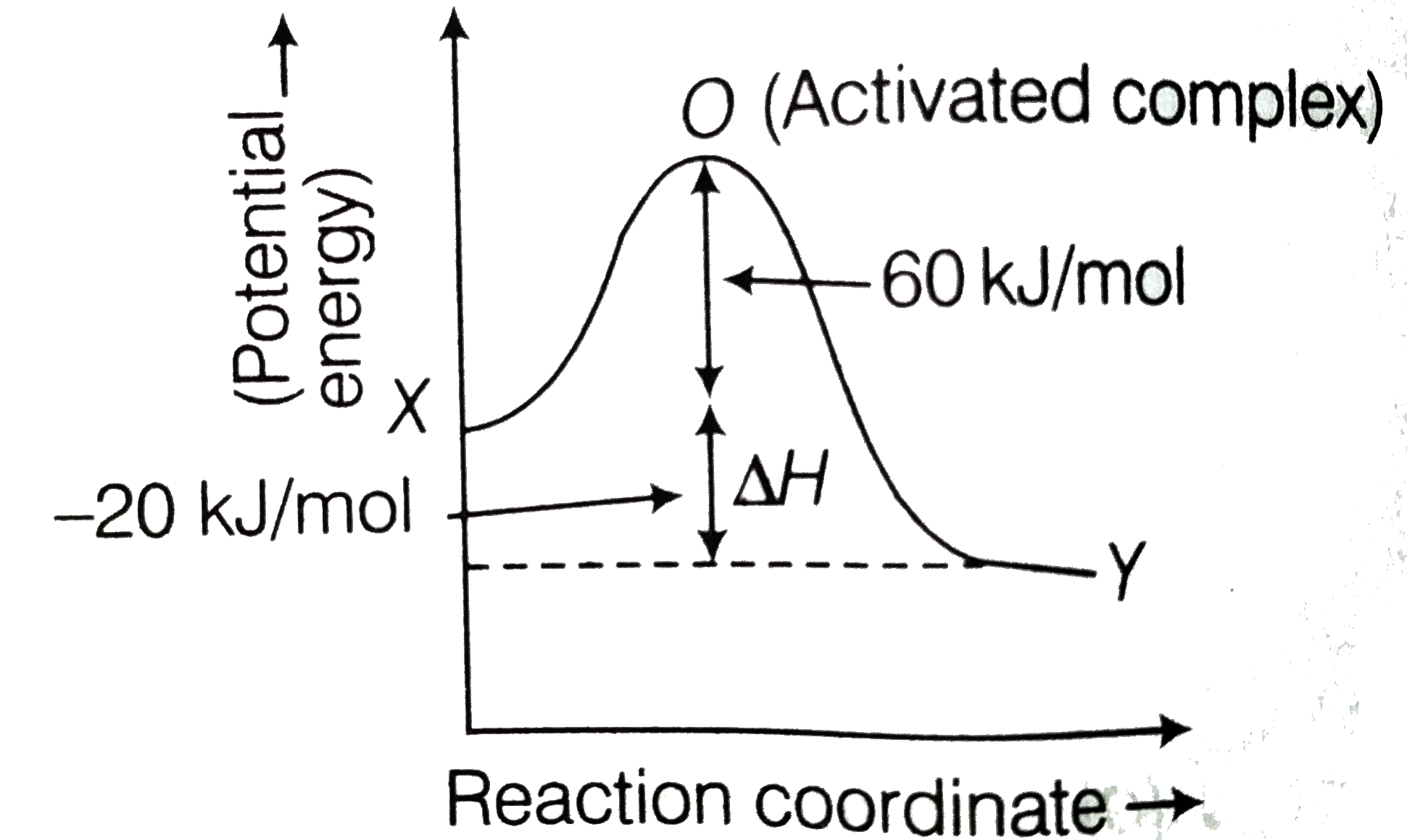
- The activation energy for the forward reaction X rarr Y is 60 kJ mol^(...
Text Solution
|
- Which among the following will show anisotropy ?
Text Solution
|
- According to the adsorption theory of catalysis, the speed of the reac...
Text Solution
|
- Among the members of VA group (N,P,As,Sb and Bi) which of the followin...
Text Solution
|
- Highest density will be of this element
Text Solution
|
- The electron affinity values (in kJ mol^(-1) ) of three halogens, X, Y...
Text Solution
|
- A suitable reagent for the distinchtion of aldehyde and ketone is
Text Solution
|
- Significance of Henry's law constant (K(H)) is
Text Solution
|
- When 1mol of a monoatomic ideal gas at TK undergoes adiabatic change u...
Text Solution
|
- The emf of the cell Zn|Zn^(2+)(1M)||Cu^(2+)|Cu(1M) is 1.1V. If the...
Text Solution
|
- The volume of 2N H(2)SO(4) solution is 0.1dm^(3). The volume of its de...
Text Solution
|
- The geometry of electron pairs around l in IF(5) is
Text Solution
|
- H(2)O is liquid while H(2)S is a gas at room temperature. Explain.
Text Solution
|
- Pick out the incorrect statement for XeF(4).
Text Solution
|
- Misch metal is an alloy of
Text Solution
|
- Carbon tetrachloride on hydrolysis with ethanolic KOH solution yields
Text Solution
|
- Cyclic trimer can be obtained as a polymerisation product by the carbo...
Text Solution
|
- Which N-atom of cyanide species is more basic?
Text Solution
|