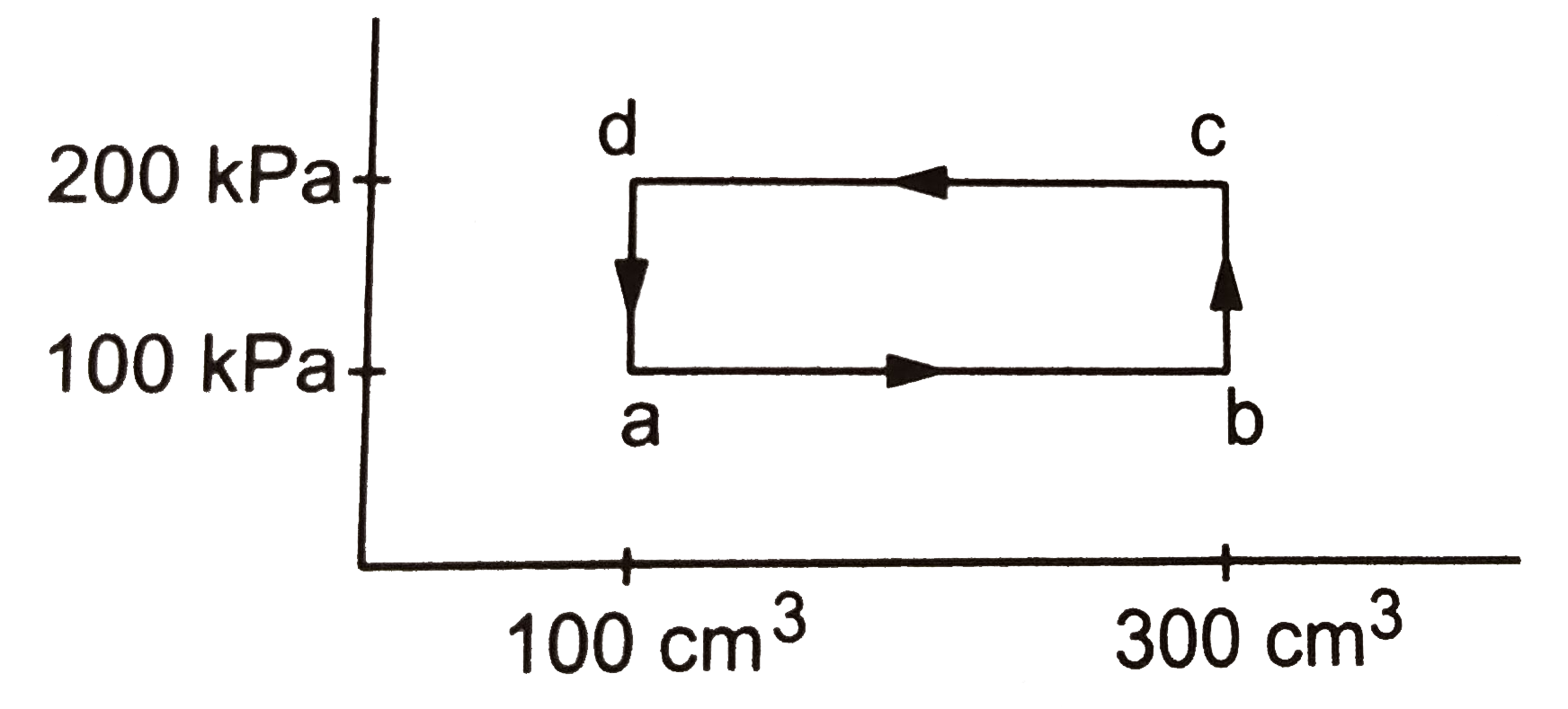
Text Solution
Verified by Experts
Topper's Solved these Questions
LAWS OF THERMODYNAMICS
HC VERMA|Exercise Objective 1|9 VideosLAWS OF THERMODYNAMICS
HC VERMA|Exercise Objective 2|5 VideosLAWS OF THERMODYNAMICS
HC VERMA|Exercise Short Answer|15 VideosKINETIC THEORY OF GASES
HC VERMA|Exercise Exercises|62 VideosNEWTON'S LAWS OF MOTION
HC VERMA|Exercise Exercises|42 Videos
Similar Questions
Explore conceptually related problems
HC VERMA-LAWS OF THERMODYNAMICS-Worked Out Examples
- A sample of an ideal gas is taken through the cyclic process abca . It...
Text Solution
|
- A thermodynamic system is taken through the cycle abcda during the par...
Text Solution
|
- calculate the increase in internal energy of 1 kg of water at 100(0)C ...
Text Solution
|
- The internal energy of a monatomic ideal gas is 1.5 nRT. One mole of h...
Text Solution
|
- A steam engin intakes 100g of steam at 100^((0))C per minute and cools...
Text Solution
|
- Shows a process ABCA performed on an ideal gas. Find the net heat give...
Text Solution
|
- consider the cyclic process ABCA on a sample of 2.0 mol of an ideal ga...
Text Solution
|
- 2.00 mol of a monatomic ideal gas (U=1.5nRT) is enclosed in an adiabat...
Text Solution
|
- A sample of an ideal gas has pressure p(0), volume V(0) and tempreture...
Text Solution
|
- A sample of 100 g water is slowly heated from 27^((0))C to 87^((0))C C...
Text Solution
|
- A heat engine operates between a cold reservoir at tempreture T(2)=300...
Text Solution
|