Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
HC VERMA-LAWS OF THERMODYNAMICS-Exercises
- A 100 kg block is started with a speed of 2.0 m s^(-1) on a long, roug...
Text Solution
|
- calculate the change in internal energy of a gas kept in a rigid conta...
Text Solution
|
- the pressure of a gas change linearly with volume from 10kPa, 200 cc t...
Text Solution
|
- An ideal gas is taken from an inirial state I to a final state f in s...
Text Solution
|
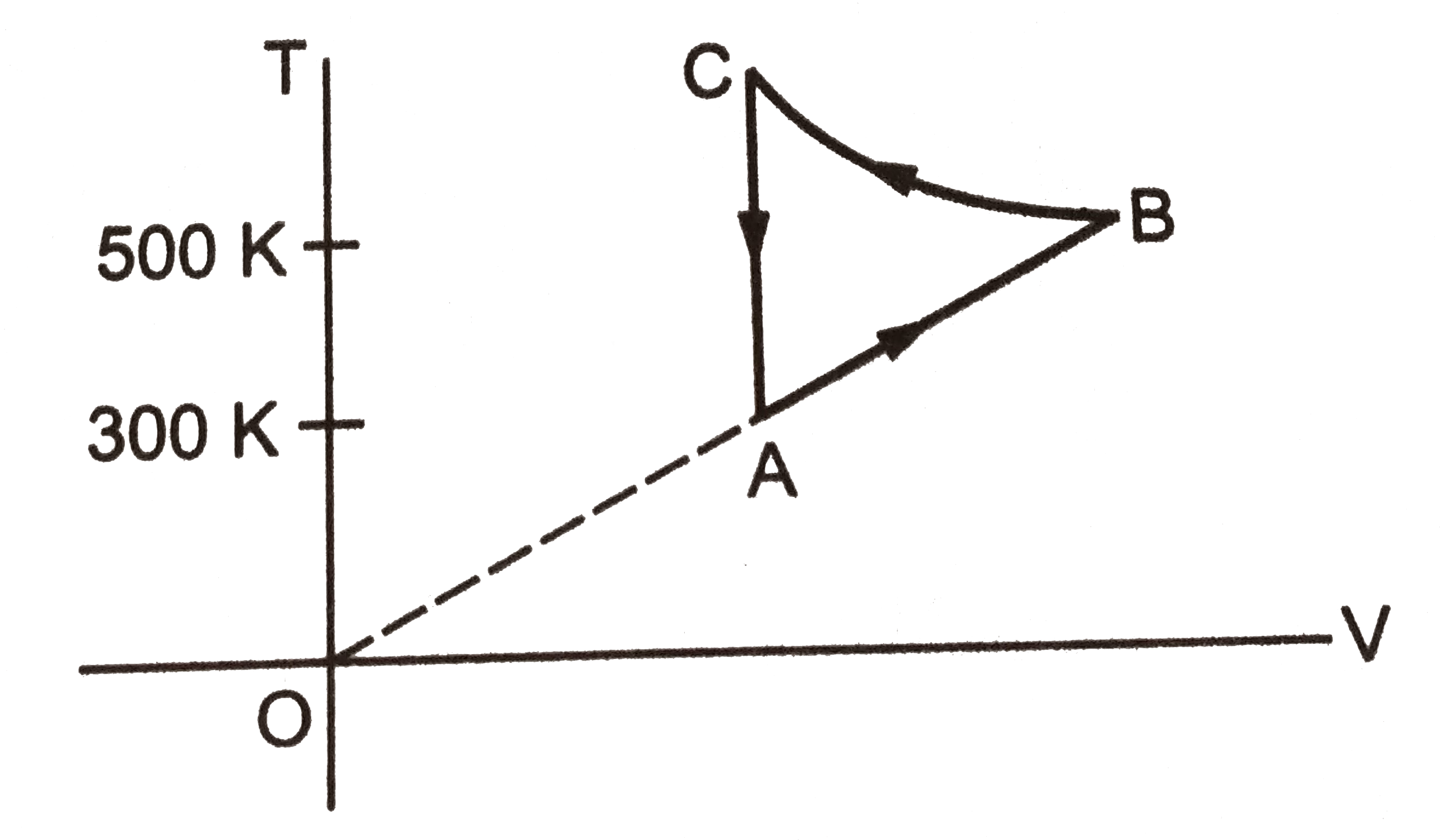
- shows three paths through which a gas can be taken from the state A to...
Text Solution
|
- when a system is taken through the process abc shown in 80 J of heat i...
Text Solution
|
- 50 eal of heat should be supplied to take a system from the state A t...
Text Solution
|
- calculate the heat absorbed by a system in going through the cyclic pr...
Text Solution
|
- A gas is taken through a cyclic process ABCA as shown in, if 2.4 cal o...
Text Solution
|
- A substance is taken through the process abc as shown in, if the inte...
Text Solution
|
- A gas is taken along the path AB as shown in , if 70 cal of heat is ex...
Text Solution
|
- The internal energy of a gas is given by U=1.5pV. It expands from 100c...
Text Solution
|
- A gas is enclosed in a cylindrical vessel fitted with a frictionless p...
Text Solution
|
- A gas is initinaly at a pressure of 100 kPa and its volume is 2.0 m^(3...
Text Solution
|
- Consider the cyclic process ABCA, shown in, performed on a sample of ...
Text Solution
|
- shows the variation in the internal energy U with the volume V of 2.0 ...
Text Solution
|
- Find the change in the internal energy of 2 kg of water as it heated f...
Text Solution
|
- Calculate the increase in the internal energy of 10 g of water when it...
Text Solution
|
- shows a cylindrial tube of volume V with adiabatic walls containing an...
Text Solution
|
- An adiabatic vessel of total volume V is divided into two equal parts ...
Text Solution
|