Text Solution
Verified by Experts
Topper's Solved these Questions
MODEL QUESTION PAPER
NAVNEET PUBLICATION - MAHARASHTRA BOARD|Exercise SECTION- C|12 VideosMODEL QUESTION PAPER
NAVNEET PUBLICATION - MAHARASHTRA BOARD|Exercise SECTION- D|5 VideosMODEL QUESTION PAPER
NAVNEET PUBLICATION - MAHARASHTRA BOARD|Exercise SECTION- D|5 VideosLAWS AND THEORIES
NAVNEET PUBLICATION - MAHARASHTRA BOARD|Exercise Assignments|2 VideosMODEL QUESTION PAPER FOR PRACTICE
NAVNEET PUBLICATION - MAHARASHTRA BOARD|Exercise SECTION-D|5 Videos
Similar Questions
Explore conceptually related problems
NAVNEET PUBLICATION - MAHARASHTRA BOARD-MODEL QUESTION PAPER -SECTION- B
- Banking of roads at curve is necessary so as to avoid
Text Solution
|
- The equation of a plane progressive wave is y=5sin2pi(8t-5x). Where y ...
Text Solution
|
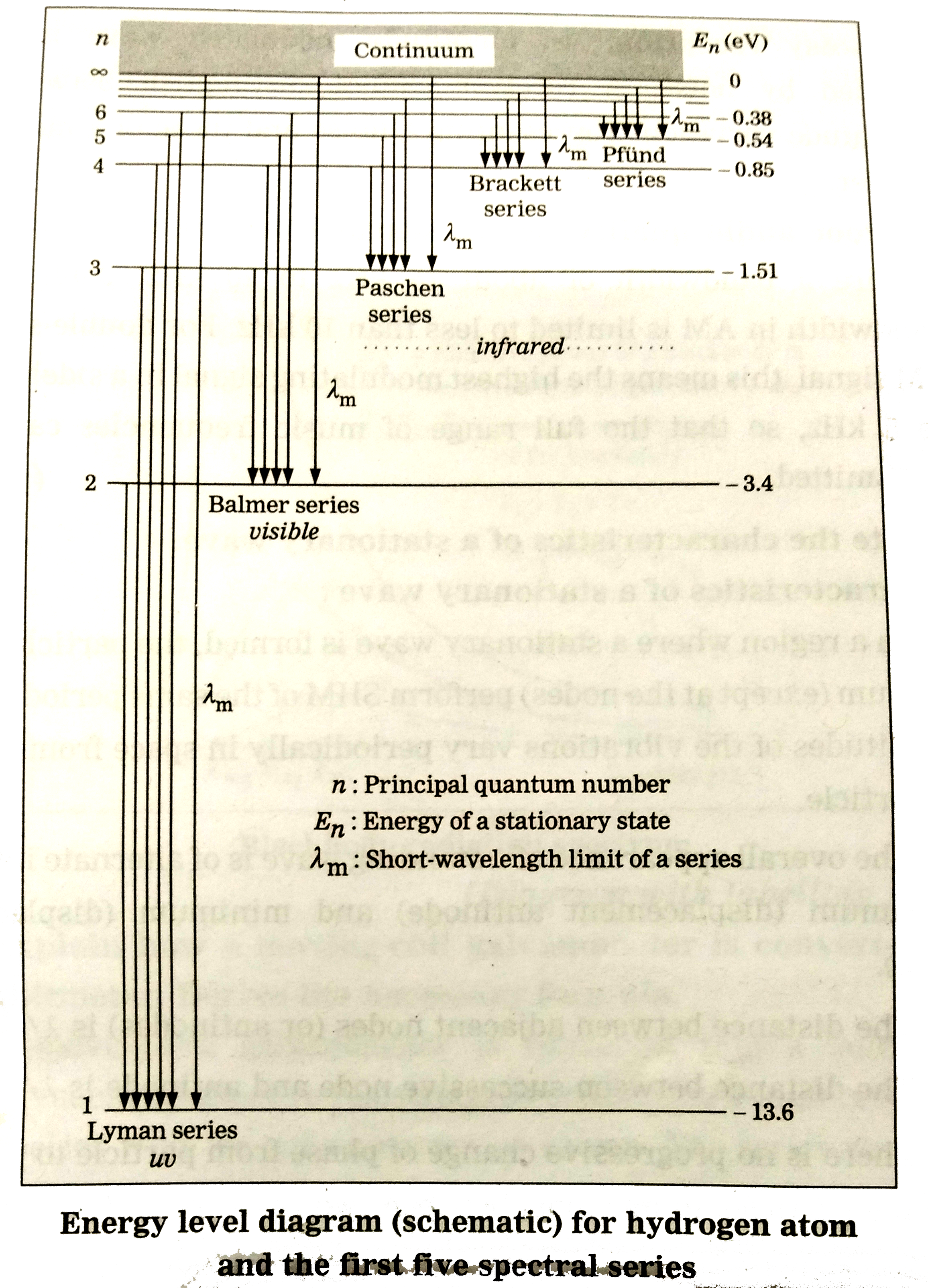
- Draw a neat, labelled energy level diagram for H atom showing the tran...
Text Solution
|
- What are the drawbacks or limitations of amplitude modulation ?
Text Solution
|
- CHARACTERISTICS OF STATIONARY WAVES
Text Solution
|
- The spectrum from a black body radiation is a
Text Solution
|
- Explain how a moving - coil galvanometer is converted into a voltmeter...
Text Solution
|
- An LCR series circuit has a resistance of 25 Omega and a reactance of...
Text Solution
|
- A solid sphere of mass 1 kg rolls on a table with linear speed 2 m/s. ...
Text Solution
|
- A potentiometer wire , 4 m long and having a resistance of 10 Omega, i...
Text Solution
|
- The total energy of a body of mass 100 g performing SHM is 0.2 J. Find...
Text Solution
|
- When a thin flake of mica (n(m) = 1.58) covers one slit of a double - ...
Text Solution
|