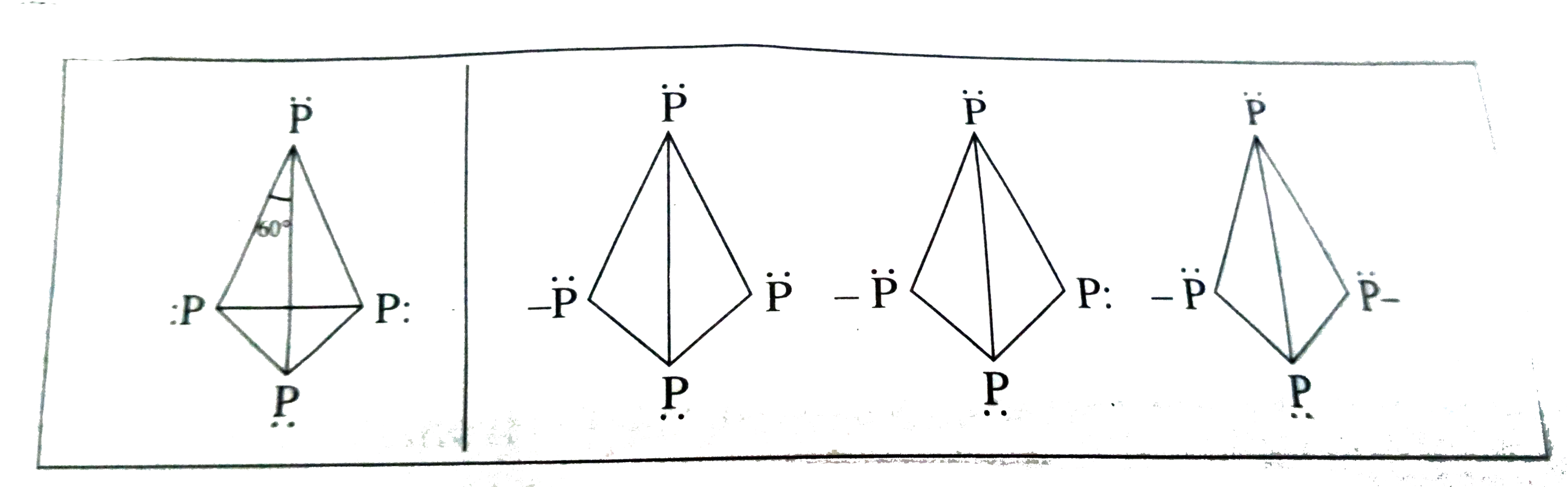
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- White phosphorus has lower melting point then red phosphours. Explain ...
Text Solution
|
- Which of the following is true for white and red phosphours except tha...
Text Solution
|
- Explain the high reactivity of white phosphorus as compared to red pho...
Text Solution
|
- Red and white phosphorus will differ but not in:
Text Solution
|
- Draw the structures of white phosphours and red phosphours. Which one ...
Text Solution
|
- Red and white phosphorus are similar in
Text Solution
|
- (i) white phosphorus (ii) red phosporus and (iii) balck phosphorus. Wr...
Text Solution
|
- सफेद फॉस्फोरस से लाल फॉस्फोरस कैसे प्राप्त किया जाता है ? लाल फॉस्फोर...
Text Solution
|
- White phosphorus has lower melting point then red phosphours. Explain ...
Text Solution
|