Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
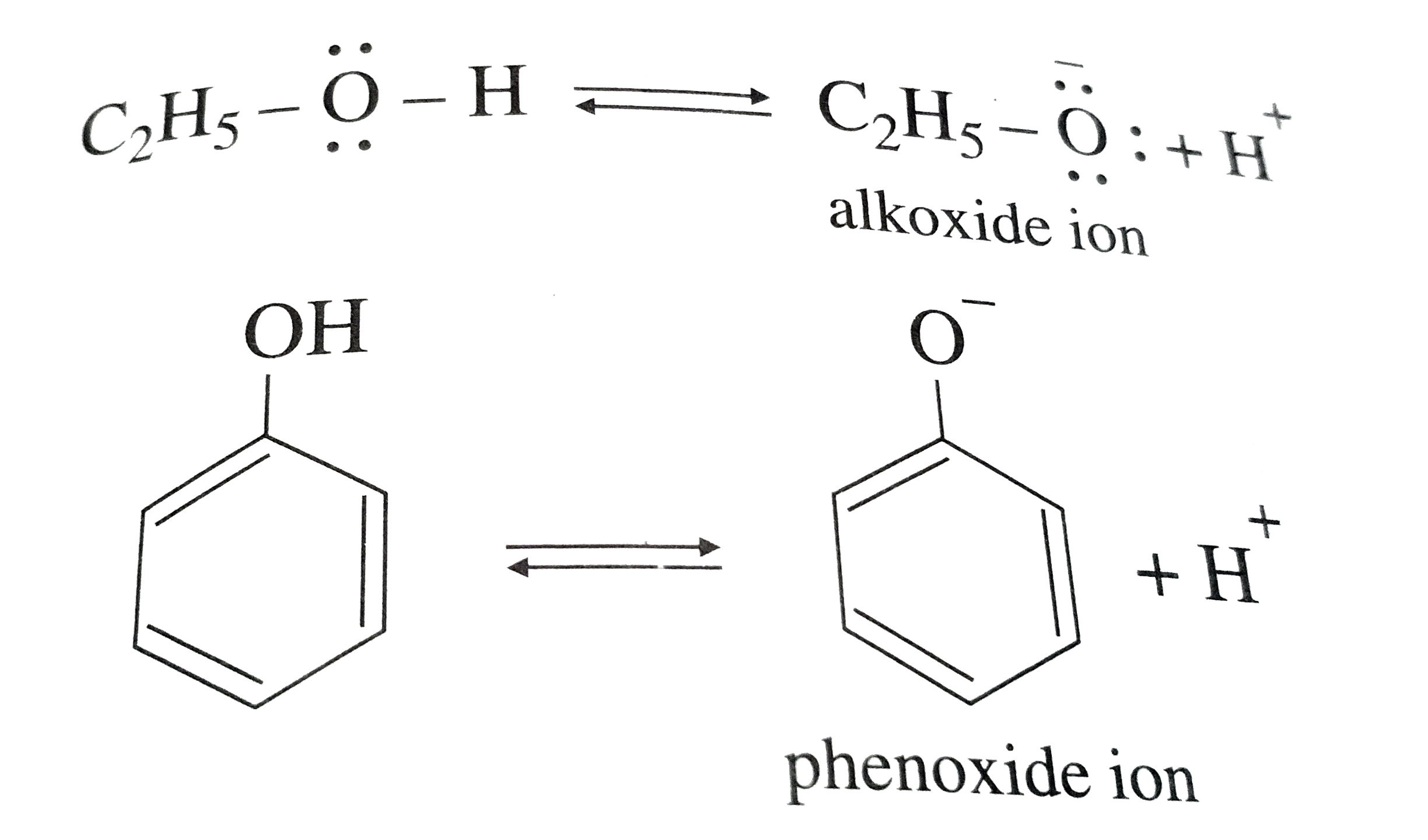
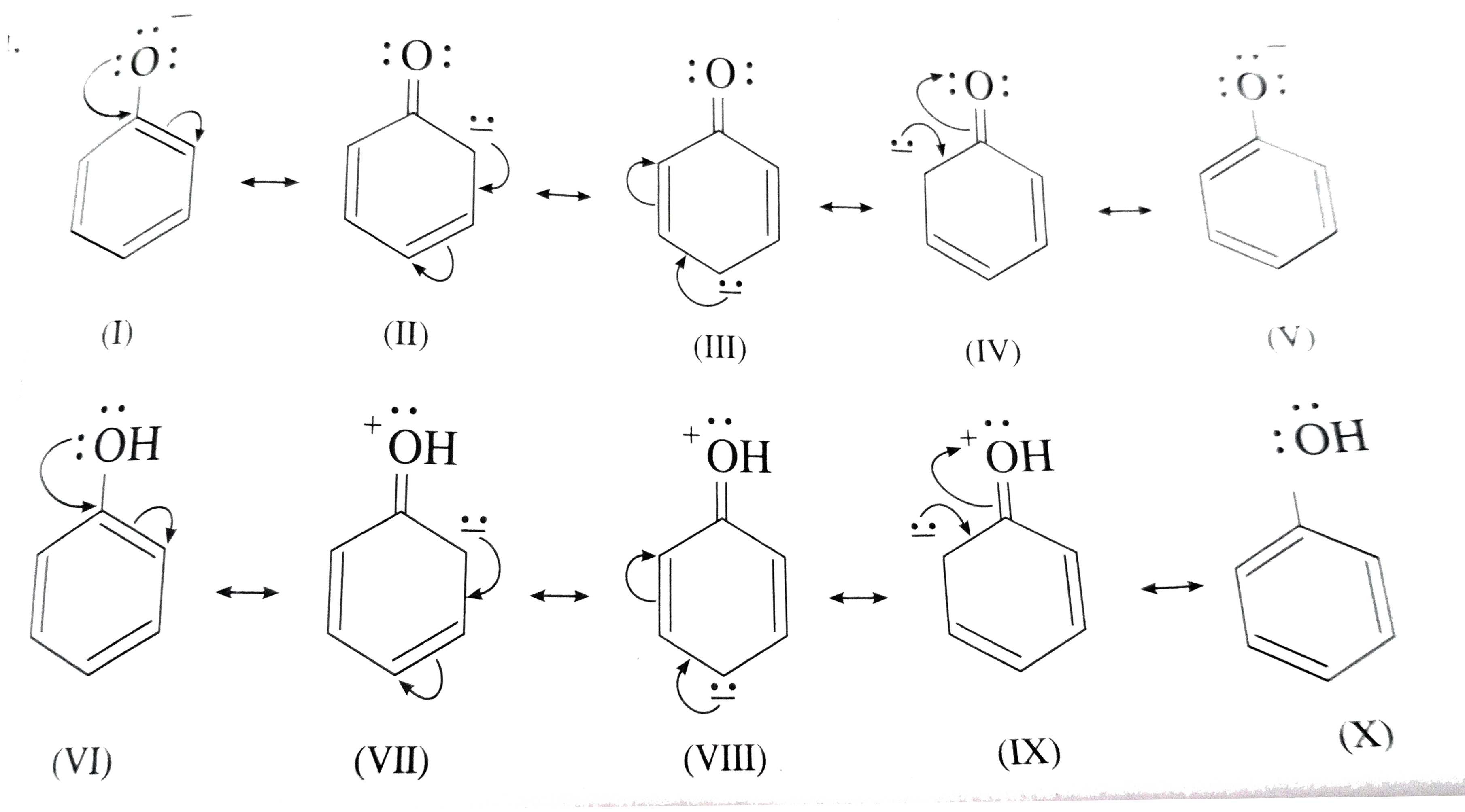
- Why is phenol more acidic than ethanol ?
Text Solution
|
- Why is phenol more acidic than ethanol ?
Text Solution
|
- Phenol is more acidic than ethanol due to
Text Solution
|
- आप ऐसी दो अभिक्रियाएं दीजिए जिनसे फीनॉल की अम्लीय प्रकृति प्रदर्शित हो...
Text Solution
|
- निम्नलिखित के कारण बतायें - (a) फेनॉल इथेनॉल से अधिक अम्लीय है। (b...
Text Solution
|
- Phenol is more acidic than ethanol.
Text Solution
|
- Assertion: Phenol undergoes Kolbe's reaction but ethanol does not. ...
Text Solution
|
- फिनॉल, ऐथेनॉल की तुलना में अधिक अम्लीय है, निम्न के कारण
Text Solution
|
- Phenol is more acidic and ethanol is less acidic than water. Why ?
Text Solution
|