Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
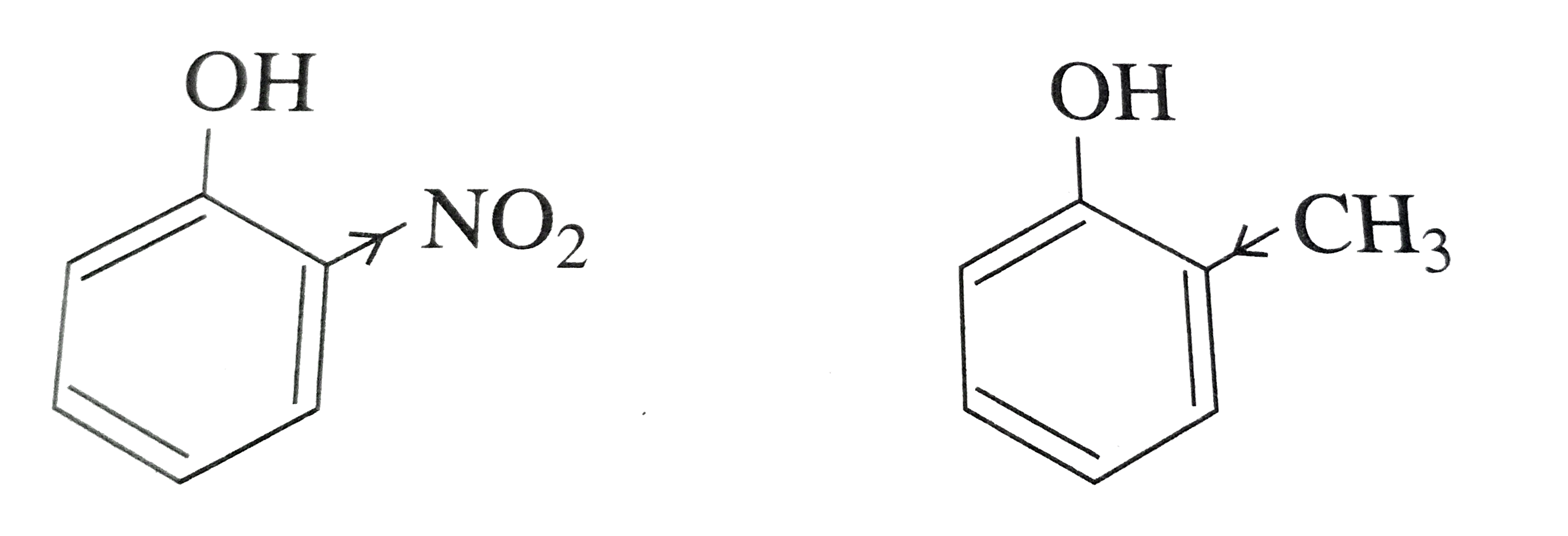
- Out of o-nitrophenol and o-cresol which is more acidic ?
Text Solution
|
- How many of the compounds are more acidic than phenol? O-cresol, m- ...
Text Solution
|
- Out of o-nitrophenol and p-nitrophenol, which is more volatile ? Expla...
Text Solution
|
- Out of o-nitrophenol and o-cresol which is more acidic ?
Text Solution
|
- Out of o-nitrophenol annd p-nitrophenol, which is more volatile? Expla...
Text Solution
|
- Out of o-nitrophenol and o-cresol which is more acidic ?
Text Solution
|
- Arrange the given compouns in decreasing order of acidity and give a s...
Text Solution
|
- Out of o-nitrophenol and p-nitrophenol, which is more volatile ?
Text Solution
|
- Out of o-nitrophenol and o-cresol which is more acidic ?
Text Solution
|