Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
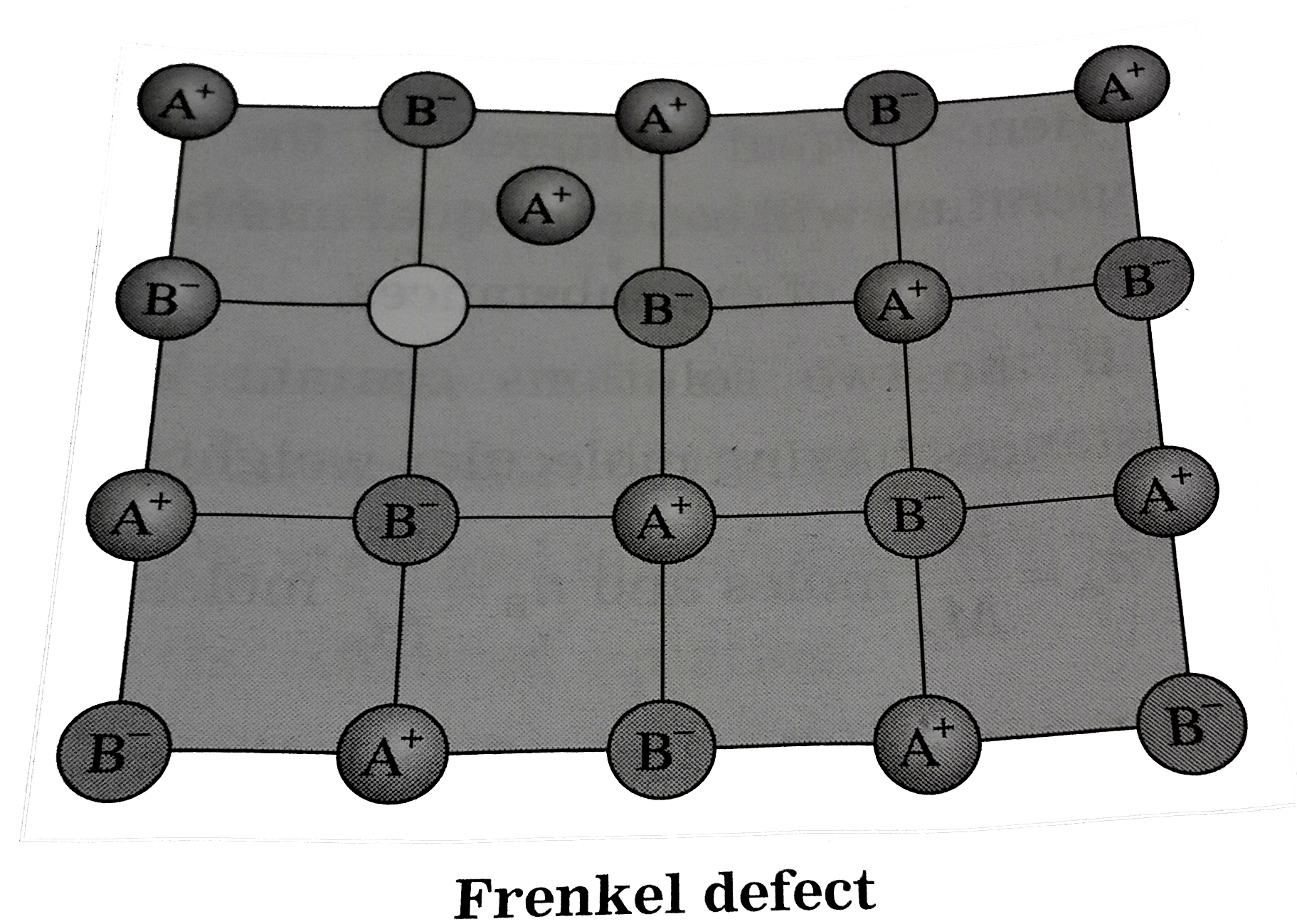
- Write a note on Frenkel defect.
Text Solution
|
- Write notes on : (i) Frenkel defects (ii) Schottky defect.
Text Solution
|
- Write the difference between Frenkel and Schottky defects.
Text Solution
|
- Write a note on Frenkel defect.
Text Solution
|
- Write a note on Frenkel defect.
Text Solution
|
- In Frenkel defect
Text Solution
|
- Write a note on Frenkel defect.
Text Solution
|
- Frenkel defect is the
Text Solution
|
- Frenkel defects are
Text Solution
|