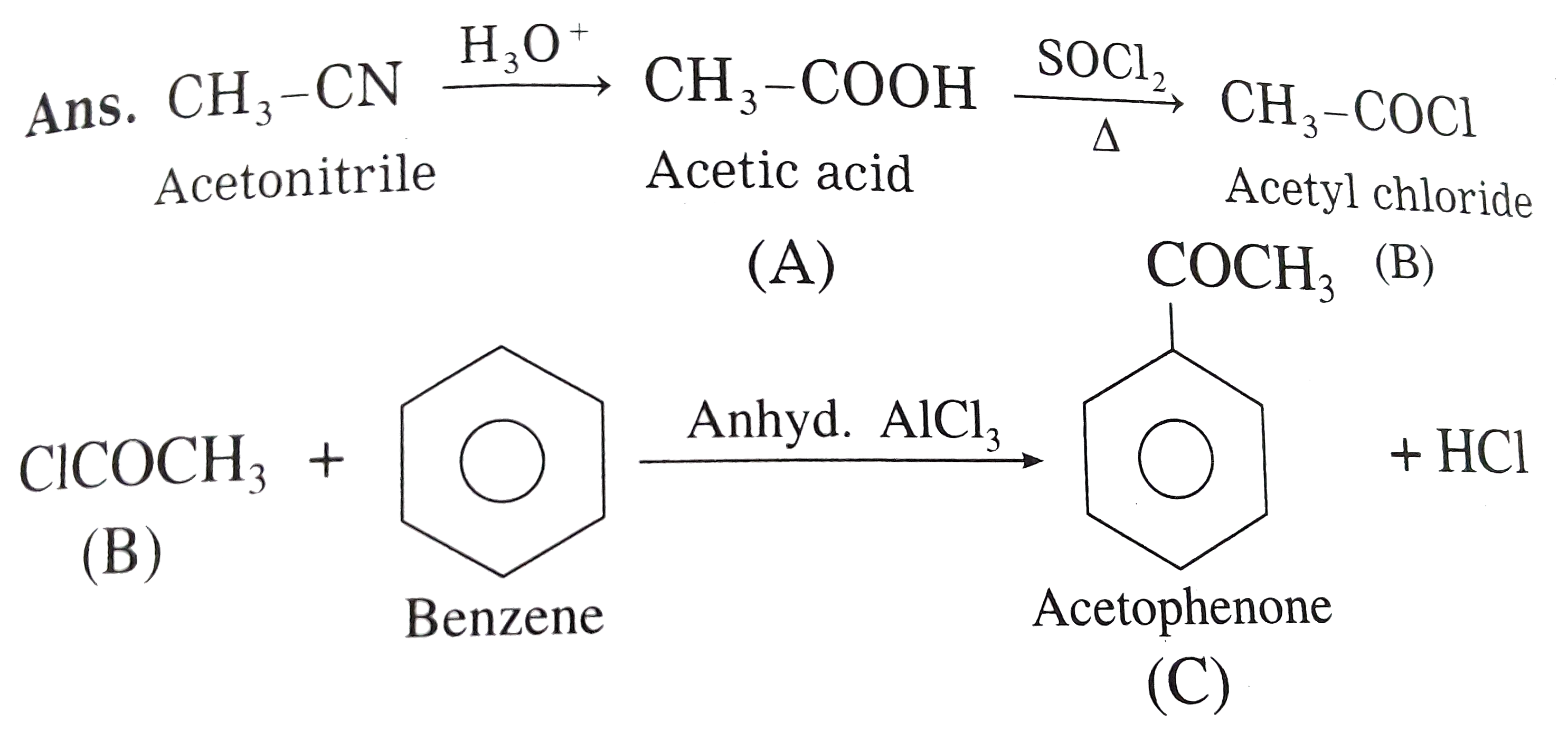
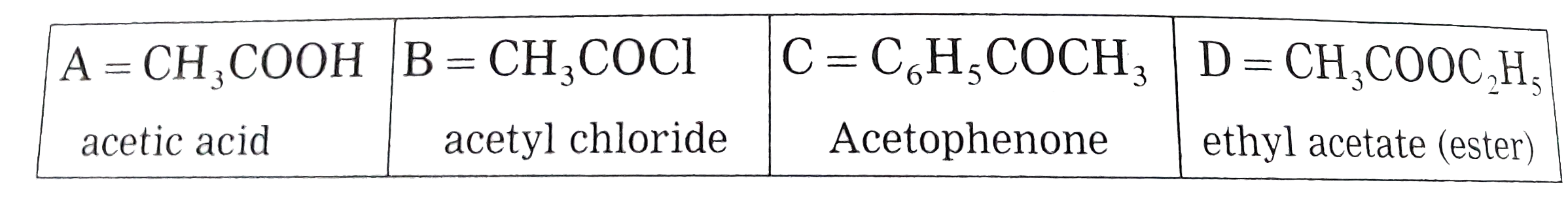
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- A nitraite on acid hydrolysis gives compound A, which reacts with thio...
Text Solution
|
- A compound (A) of boron reacts with Nme(3) to give an adduct (B) which...
Text Solution
|
- An organic compound [A] is C(5)H(8)O(3). On heating with soda lime, it...
Text Solution
|
- An organic compound [A] is C(5)H(8)O(3). On heating with soda lime, it...
Text Solution
|
- A compound (A) of boron reacts with Nme(3) to give an adduct (B) which...
Text Solution
|
- A compound (A) of boron reacts with NMe(3) to give an adduct (B) which...
Text Solution
|
- A nitraite on acid hydrolysis gives compound A, which reacts with thio...
Text Solution
|
- Compound A (ester ) reacts with LiAlH4 gives B and C. Compound B on ox...
Text Solution
|
- Compound 'A' react with CH3-Cl gives B. B react with dil. H2SO4 gives ...
Text Solution
|