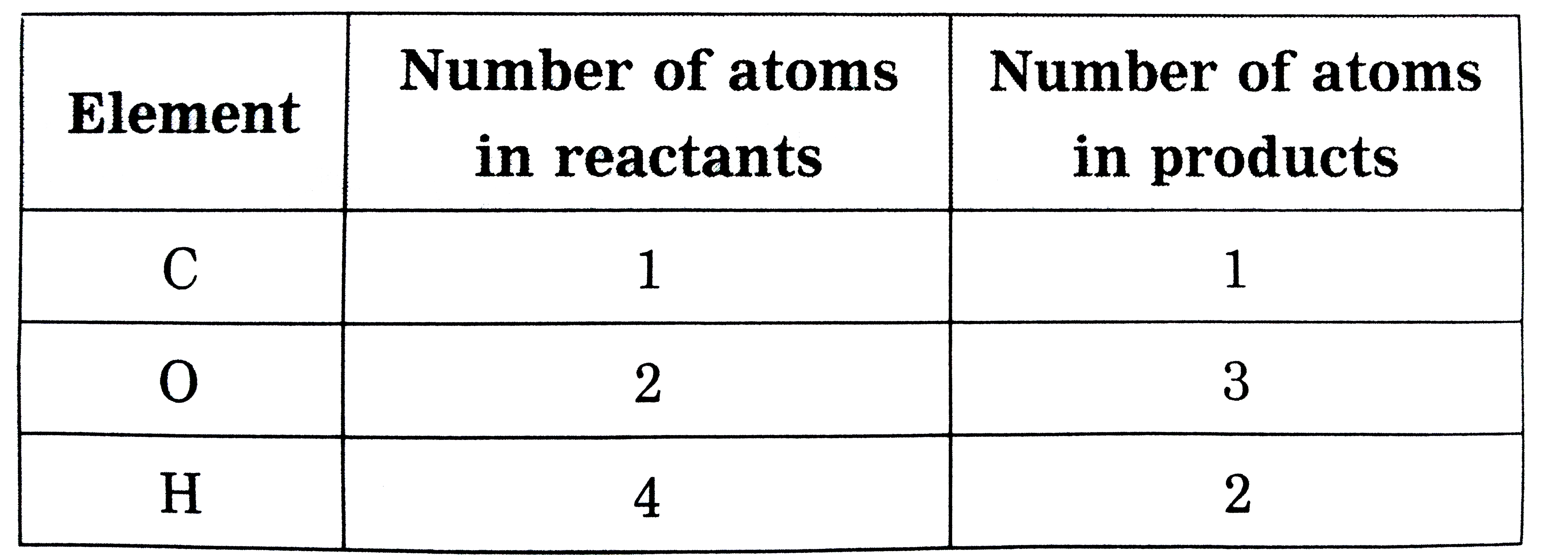
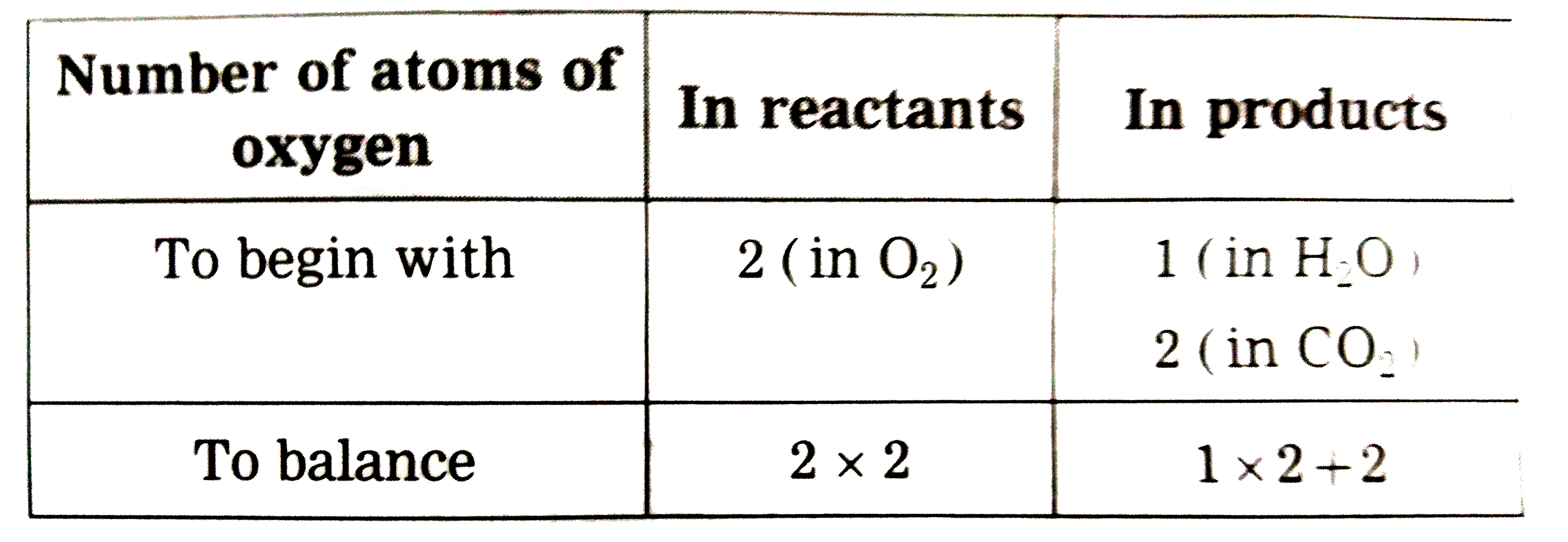Text Solution
Verified by Experts
Topper's Solved these Questions
MODEL ACTIVITY SHEET
NAVNEET PUBLICATION - MAHARASHTRA BOARD|Exercise ANSWER THE FOLLOWING QUESTIONS|1 VideosMODEL ACTIVITY SHEET
NAVNEET PUBLICATION - MAHARASHTRA BOARD|Exercise ANSWER THE FOLLOWING|5 VideosMISCELLANEOUS QUESTIONS
NAVNEET PUBLICATION - MAHARASHTRA BOARD|Exercise Assignment|2 VideosMODEL ACTIVITY SHEET FOR PRACTICE
NAVNEET PUBLICATION - MAHARASHTRA BOARD|Exercise Exercise|18 Videos
Similar Questions
Explore conceptually related problems
NAVNEET PUBLICATION - MAHARASHTRA BOARD-MODEL ACTIVITY SHEET -ANSWER THE FOLLOWING
- Write the balanced equations for the following reactions : CH(4)+O(2...
Text Solution
|
- Explain the meaning of symbols A and B.
Text Solution
|
- In case of this disaster what will your pre and post disaster mana...
Text Solution
|
- Reorganize the following food chain. Describe the ecosystem to which i...
Text Solution
|
- Observe the diagram given below and answer the questions : ...
Text Solution
|
- Observe the diagram given below and answer the questions : ...
Text Solution
|