Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
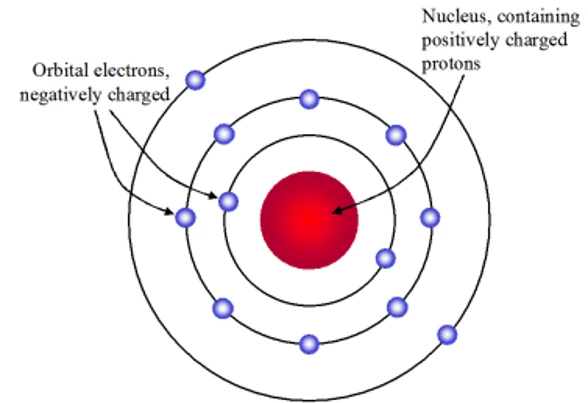
- Rutherford Atomic Model
Text Solution
|
- Rutherford Atomic Model
Text Solution
|
- Describe Rutherford atom model. What are the drawbacks of this model?
Text Solution
|
- Rutherford Model Of Atom
Text Solution
|
- Postulate Of Rutherford Atomic Model
Text Solution
|
- Thomson Model Of Atom | Rutherford Model Of Atom
Text Solution
|
- రూథర్ఫోర్డ్ పరమాణు నమూనాను ఏమని పిలుస్తారు?
Text Solution
|
- Describe Rutherford atom model. What are the drawbacks of this model?
Text Solution
|
- Explain Rutherford nuclear model of atom
Text Solution
|