Similar Questions
Explore conceptually related problems
Recommended Questions
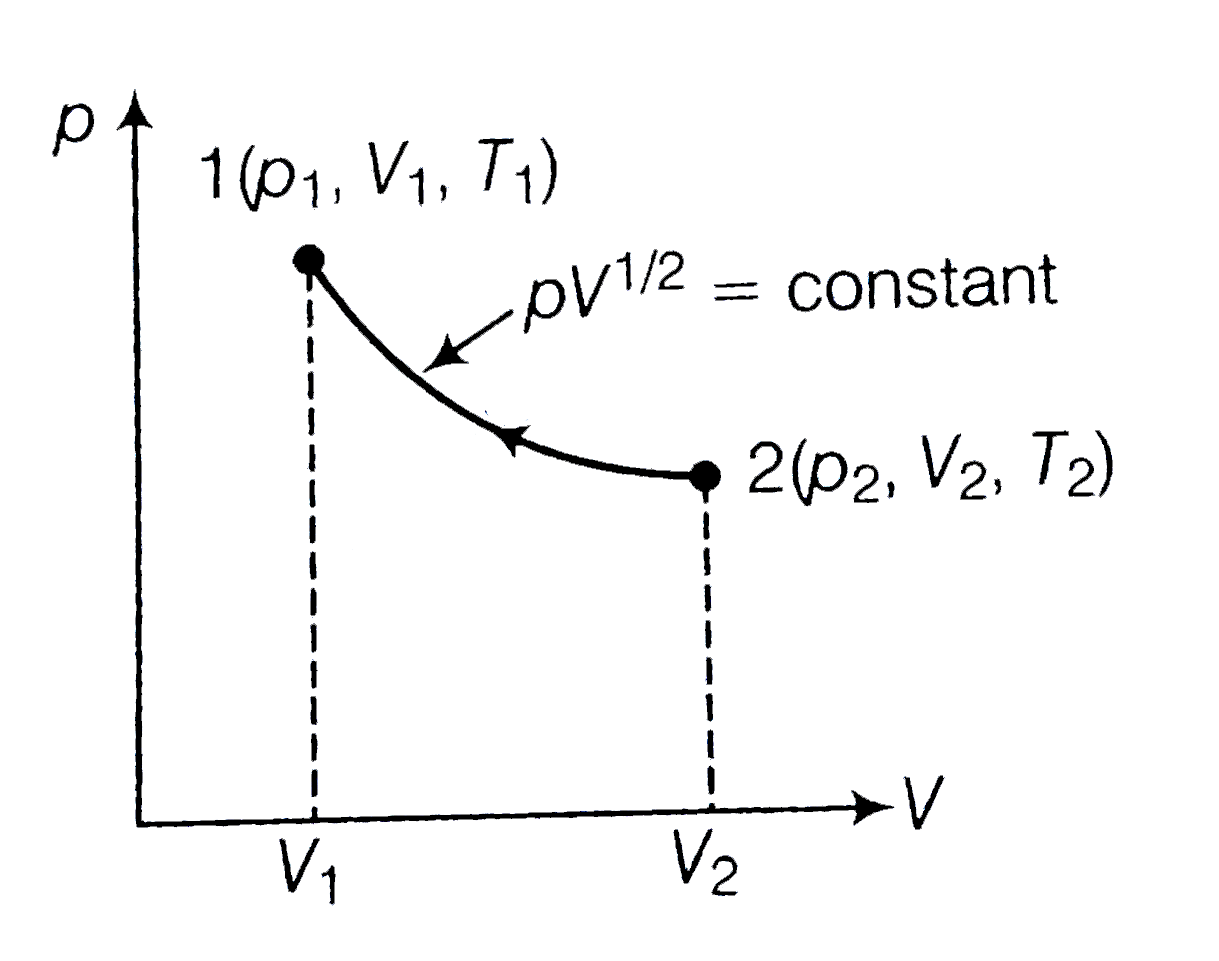
- Consider a p-V diagram in which the path followed by one mole of perfe...
Text Solution
|
- Consider a P-V diagram in which the path followed by one mole of perfe...
Text Solution
|
- n moles of an ideal gas is taken through a four step cyclic process as...
Text Solution
|
- One mole of an ideal gas at a temperature T(1) expands according to th...
Text Solution
|
- The P-V diagram of path followed by one mole of perfect gas in a cylin...
Text Solution
|
- 1 mole of an ideal gas in a cylindrical container have the P-V diagram...
Text Solution
|
- One mole of an ideal gas at temperature T(1) expends according to the ...
Text Solution
|
- One mole of an ideal gas at temperature T(1) expands according to the ...
Text Solution
|
- Consider a P-V diagram in which the path followed by one mole of perfe...
Text Solution
|