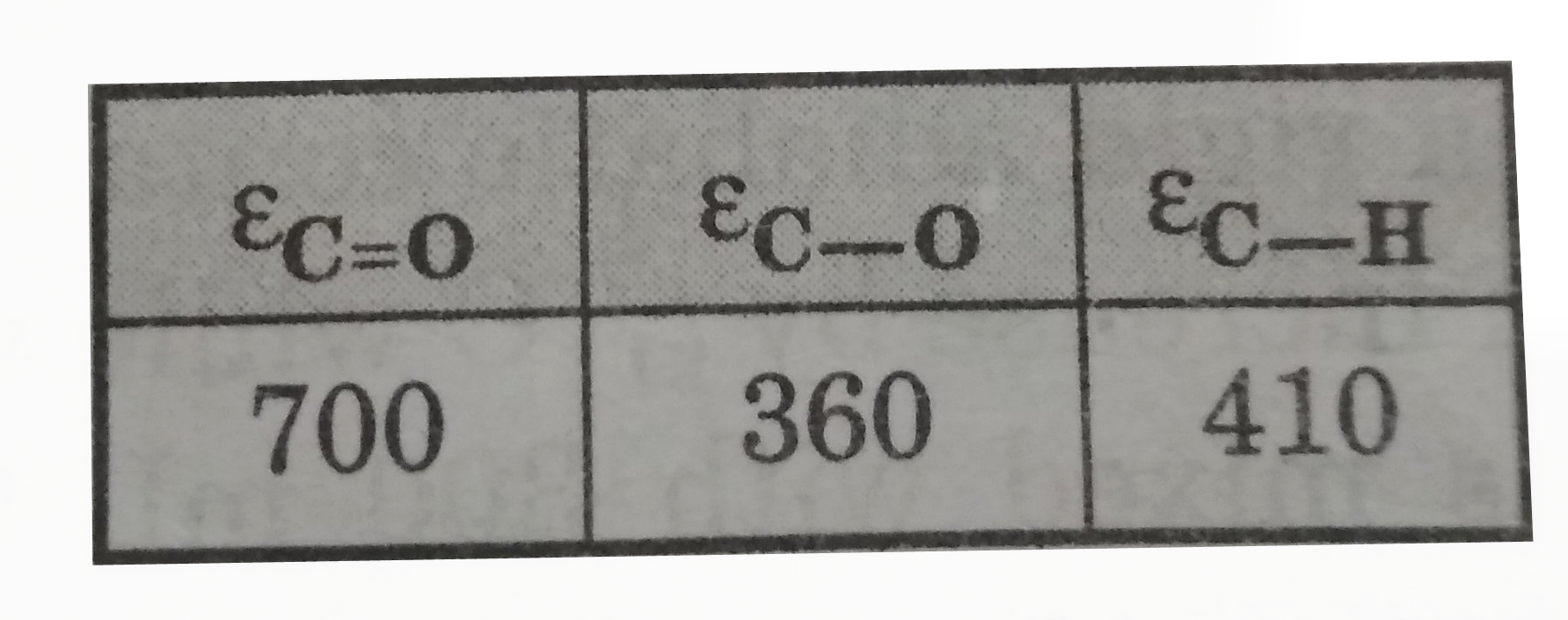
Similar Questions
Explore conceptually related problems
Recommended Questions
- The enthalpy of gas phase trimerzation of one mole of gaseous formalde...
Text Solution
|
- Using the bond enthalpy data given below, calculate the enthalpy of fo...
Text Solution
|
- The bond energies of C=C and C-C at 298 K are 590 and 331 kJ mol^(-1) ...
Text Solution
|
- Calculate C-H bond energy from the following data : Delta(f)H" "[C(g)]...
Text Solution
|
- Molar enthalpy of combustion of C(2)H(2)(g),C("graphite")andH(2)(g) ar...
Text Solution
|
- The enthalpy of gas phase trimerzation of one mole of gaseous formalde...
Text Solution
|
- Calculate the enthalpy of isomerization of ethanol to dimethy ether: ...
Text Solution
|
- Calculate enthalpy change (in Kj) when 2 mole of liquid acetic acid un...
Text Solution
|
- The reaction CH(4) (g) + Cl(2) (g) rarr CH(3)Cl (g) + HCl (g) has Delt...
Text Solution
|