Wave property of electron implies that they will show diffraction effected . Davisson and Germer demonstrated this by diffracting electron from crystals . The law governing the diffraction from a crystals is obtained by requiring that electron waves reflected from the planes of atoms in a crystal inter fere constructiely
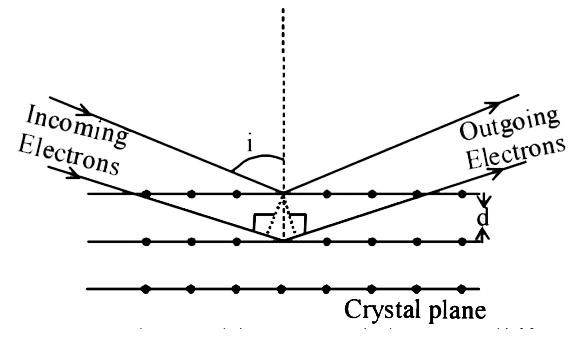
If a strong diffraction peak is observed when electrons are incident at an angle `i` from the normal to the crystal planes with distance `d` between them (see fig) de Brogle wavelength `lambda_(dB)` of electrons can be calculated by the relationship (n is an intenger)