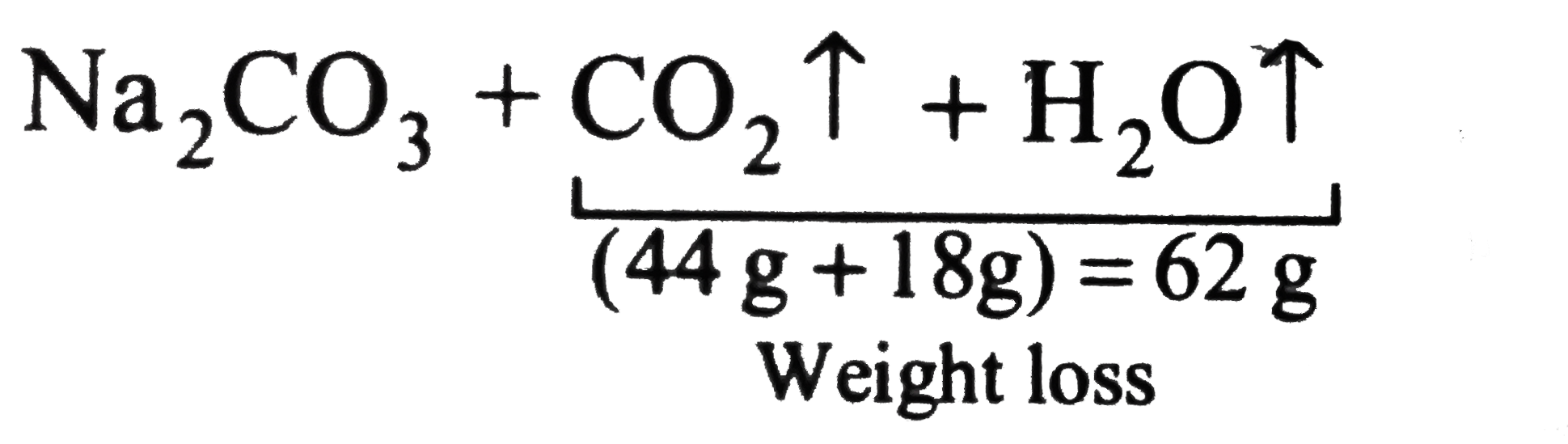Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOME BASIC CONCEPTS AND MOLE CONCEPT
CENGAGE CHEMISTRY|Exercise Ex 1.1 Objective Questions (Single Correct)|3 VideosSOME BASIC CONCEPTS AND MOLE CONCEPT
CENGAGE CHEMISTRY|Exercise Ex 1.1 Fill In The Blanks|1 VideosSOME BASIC CONCEPTS AND MOLE CONCEPT
CENGAGE CHEMISTRY|Exercise Archives Subjective|11 VideosS-BLOCK GROUP 2 - ALKALINE EARTH METALS
CENGAGE CHEMISTRY|Exercise Ex 5.1 Objective|2 VideosSTATES OF MATTER
CENGAGE CHEMISTRY|Exercise Exercises (Ture False)|25 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-SOME BASIC CONCEPTS AND MOLE CONCEPT-Ex 1.1 Objective Questions
- Objective question (only one correct). I. Which of the following has...
Text Solution
|
- A metal M of atomic weight 54.9 has a density of 7.42 g cm^(-3). Calcu...
Text Solution
|
- Fil in the blanks a. The mass of 1 molecule of water (H(2) O) is ………...
Text Solution
|
- Objective question . i. A certains compound has the molecular formul...
Text Solution
|
- A 2.0 g of mixture of Na(2)CO(3) and NaHCO(3) loses 0.248 g when heate...
Text Solution
|
- Fill in the blanks. a. The equivalent weight of NaHCO(3) is ……..and ...
Text Solution
|