A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
NEET
NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)|Exercise QUESTION|92 VideosNEET
NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)|Exercise MCQ|94 VideosNEET
NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)|Exercise MCQ|94 VideosMETALLURGICAL OPERATIONS
NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)|Exercise MCQ|21 VideosNEET 2018
NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)|Exercise QUESTION|45 Videos
Similar Questions
Explore conceptually related problems
NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)-NEET-CHEMISTRY
- For the cell reaction: 2Fe^(3+)(aq)+2l^(-)(aq)to2Fe^(2+)(aq)+l(2)(aq...
Text Solution
|
- The compound that is most difficult protonate is
Text Solution
|
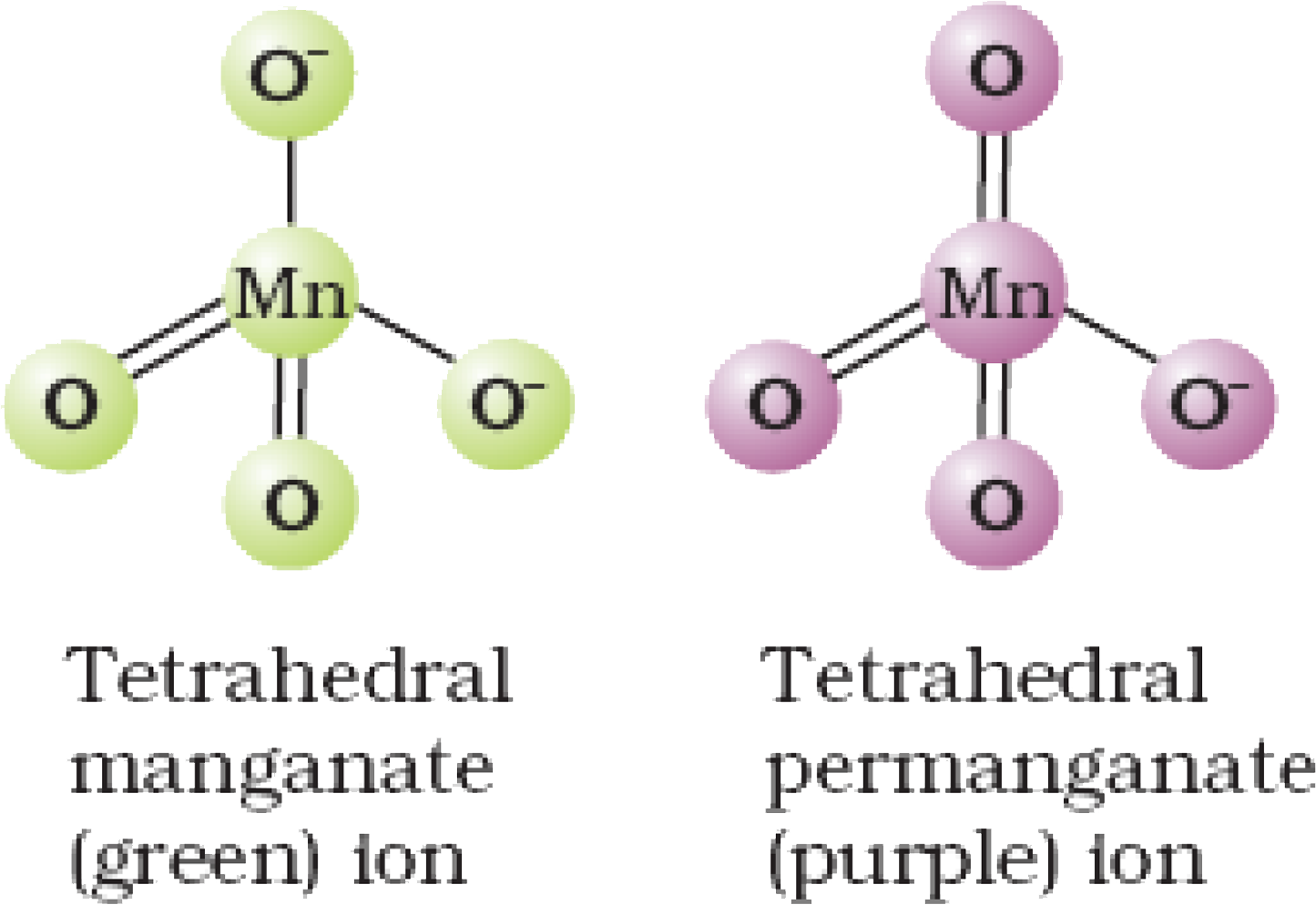
- The magnitude and permanganate ions are tetrahedral due to
Text Solution
|
- The correct order of the basic strength of methyl substituted amines i...
Text Solution
|
- An alkene "A" on reaction with O3 and Zn gives propanone and ethanol i...
Text Solution
|
- For the second period elements the correct increasing order of first i...
Text Solution
|
- A gas at 350 K and 15 bar has molar volume 20 percent smaller than tha...
Text Solution
|
- For a cell involving one electron E(cell)^(0)=0.59V and 298K, the equi...
Text Solution
|
- Which will make basic buffer?
Text Solution
|
- Which is the correct thermal stability order of H(2)E(E=O,S,Se,Te and ...
Text Solution
|
- For an ideal solution, the correct option is:
Text Solution
|
- The biodegradable polymer is :
Text Solution
|
- Enzymes that utilize ATP in phosphate transfer require an alkaline era...
Text Solution
|
- If the rate constant for a first order reaction is k, the time (t) req...
Text Solution
|
- Which of the following diatomic molecular species has only pi bonds ac...
Text Solution
|
- pH of a saturated solution of Ca(OH)(2) is 9. the solubility product (...
Text Solution
|
- The mixture that forms maximum boiling azeotrope is :
Text Solution
|
- 4d, 5p, 5f and 6p orbitals are arranged in the order of decreasing ene...
Text Solution
|
- Which of the following is an amphoteric hydroxide
Text Solution
|
- Which of the following is incorrect statement?
Text Solution
|