Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
LAWS OF THERMODYNAMICS
DC PANDEY|Exercise Level 2 More Than One Correct|6 VideosLAWS OF THERMODYNAMICS
DC PANDEY|Exercise Level 2 Passage I|2 VideosLAWS OF THERMODYNAMICS
DC PANDEY|Exercise Level 1 Subjective|24 VideosLAWS OF MOTION
DC PANDEY|Exercise Medical entrances gallery|39 VideosMAGNETIC EFFECT OF CURRENT AND MAGNETISM
DC PANDEY|Exercise Integer type Questions|10 Videos
Similar Questions
Explore conceptually related problems
DC PANDEY-LAWS OF THERMODYNAMICS-Level 2 Single Correct
- A monoatomic ideal gas, initially at temperature T1, is enclosed in a ...
Text Solution
|
- One mole of an ideal gas is taken through a cyclic process. The minimu...
Text Solution
|
- Two moles of an ideal gas are undergone a cyclic process 1-2-3-1. If n...
Text Solution
|
- Two cylinders A and B fitted with pistons contain equal amounts of an ...
Text Solution
|
- A gas follows a process TV^(n-1)=const ant, where T= absolute temperat...
Text Solution
|
- One mole of an ideal gas at temperature T expands slowly according to ...
Text Solution
|
- 600J of heat is added to a monoatomic gas in a process in which the ga...
Text Solution
|
- The internal energy of a gas is given by U=2pV. It expands from V0 to ...
Text Solution
|
- The figure shows two paths for the change of state of a gas from A to ...
Text Solution
|
- p-T diagram of one mole of an ideal monatomic gas is shown. Processes ...
Text Solution
|
- An ideal monoatomic gas undergoes a process in which its internal ener...
Text Solution
|
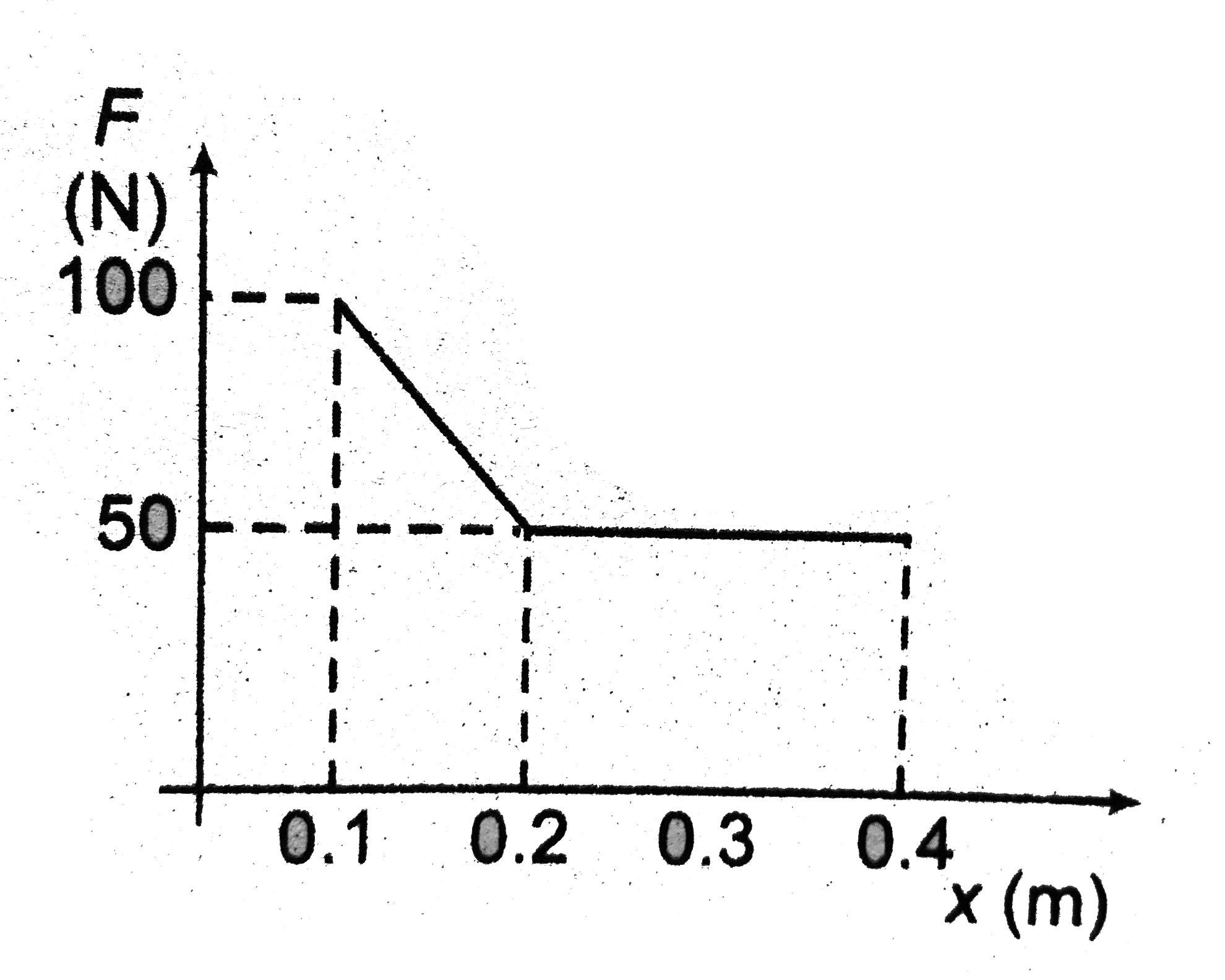
- The given figure shows the variation of force applied by ideal gas on ...
Text Solution
|
- A gas can expand through two processes : (i) isobaric, (ii) p/V =const...
Text Solution
|
- An ideal gas of adiabatic exponent gamma is expanded so that the amoun...
Text Solution
|
- A thermodynamical process is shown in the figure with pA=3xxp(atm), VA...
Text Solution
|
- A gas takes part in two processes in which it is heated from the same ...
Text Solution
|
- A closed system receives 200kJ of heat at constant volume. It then rej...
Text Solution
|
- 100 moles of an ideal monatomic gas undergoes the thermodynamic proces...
Text Solution
|
- Two moles of an ideal monoatomic gas are expanded according to the equ...
Text Solution
|
- The state of an ideal gas is changed through an isothermal process at ...
Text Solution
|