Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CALORIMETRY & HEAT TRANSFER
DC PANDEY|Exercise Example Type 3|1 VideosCALORIMETRY & HEAT TRANSFER
DC PANDEY|Exercise Example Type 4|4 VideosCALORIMETRY & HEAT TRANSFER
DC PANDEY|Exercise Example Type 1|4 VideosBASIC MATHEMATICS
DC PANDEY|Exercise Exercise|13 VideosCALORIMETRY AND HEAT TRANSFER
DC PANDEY|Exercise Medical entrance s gallery|38 Videos
Similar Questions
Explore conceptually related problems
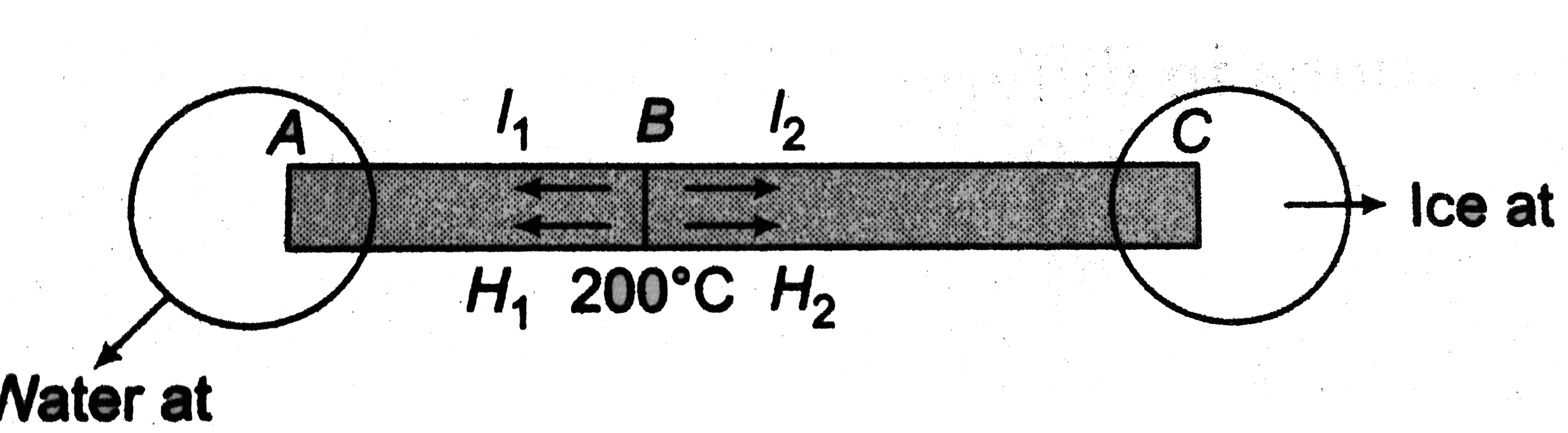 .
.