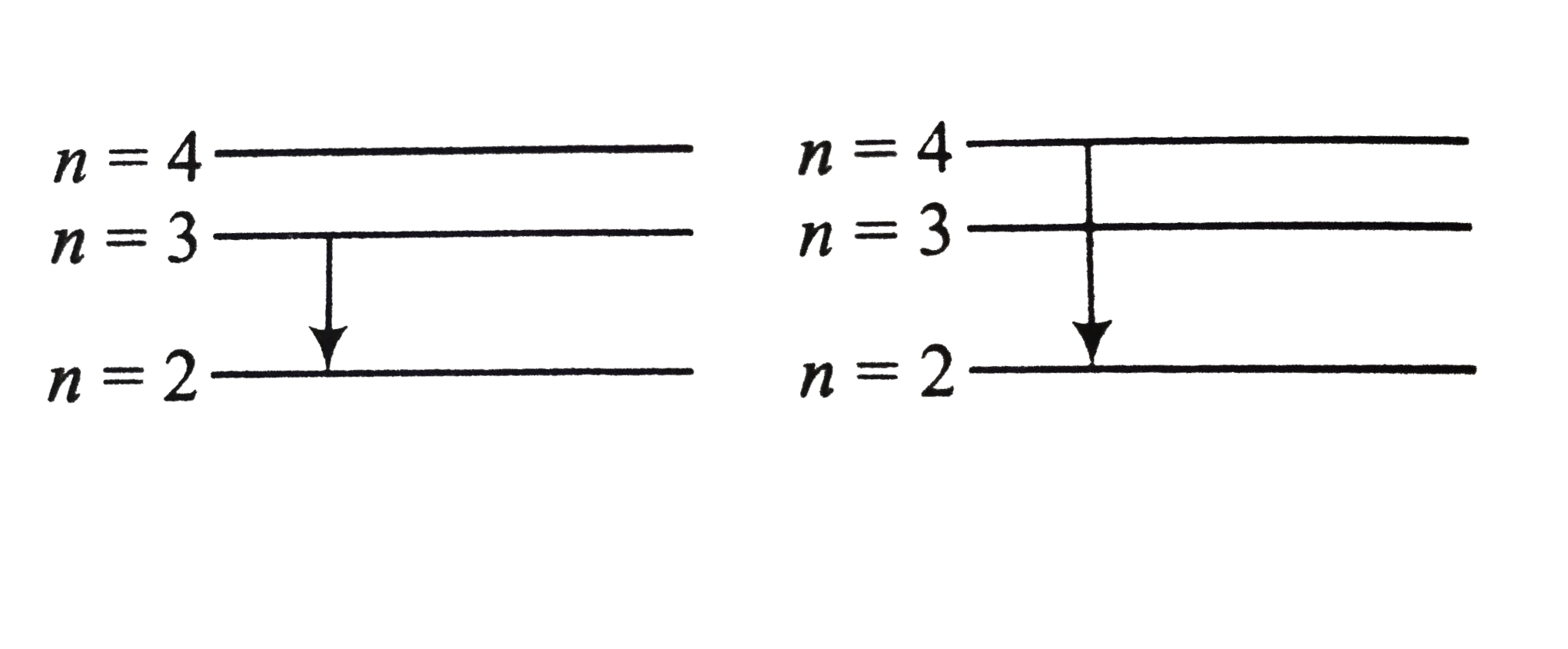
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
MODERN PHYSICS - 1
DC PANDEY|Exercise Exercise 33.3|6 VideosMODERN PHYSICS - 1
DC PANDEY|Exercise Exercise 33.4|8 VideosMODERN PHYSICS - 1
DC PANDEY|Exercise Exercise 33.1|6 VideosMODERN PHYSICS
DC PANDEY|Exercise Integer Type Questions|17 VideosMODERN PHYSICS - 2
DC PANDEY|Exercise Level 2 Subjective|10 Videos
Similar Questions
Explore conceptually related problems
DC PANDEY-MODERN PHYSICS - 1-Exercise 33.2
- Find the ionisation energy of a doubly lonized lithium atom.
Text Solution
|
- A hydrogen atom is in a state with energy -1.51 eV. in the Bohr model...
Text Solution
|
- As an electron makes a transition from an excited state to the ground ...
Text Solution
|
- The wavelength of the ultraviolet region of the hydrogen spectrum is ...
Text Solution
|
- The largest wavelength in the ultraviolet region of the hydrogen spec...
Text Solution
|
- A photon collides with a stationary hydrogen atom in ground state inel...
Text Solution
|
- A hydrogen atom and a Li^(2+) ion are both in the second excited stat...
Text Solution
|
- The transition from the state n=4 to n=3 in a hydrogen like atom resul...
Text Solution
|
- As per Bohr model , the minimum energy (in eV) required to remove elec...
Text Solution
|
- Consider the spectral line resulting from the transition n = 2 rarr n...
Text Solution
|
- The energy levels of a cartain atom are shown in figure. If a photon o...
Text Solution
|
- Find the longest wavelength present in the Balmer series of hydrogen.
Text Solution
|