A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
MODERN PHYSICS - 1
DC PANDEY|Exercise Level 2 More Than One Correct|6 VideosMODERN PHYSICS - 1
DC PANDEY|Exercise Level 2 Comprehension Based|3 VideosMODERN PHYSICS - 1
DC PANDEY|Exercise Level 1 Subjective|40 VideosMODERN PHYSICS
DC PANDEY|Exercise Integer Type Questions|17 VideosMODERN PHYSICS - 2
DC PANDEY|Exercise Level 2 Subjective|10 Videos
Similar Questions
Explore conceptually related problems
DC PANDEY-MODERN PHYSICS - 1-Level 2 Single Correct
- The excitation energy of a hydrogen -like ion in its first excited sta...
Text Solution
|
- An electron in a hydrogen in a hydrogen atom makes a transition from f...
Text Solution
|
- In a sample of hydrogen like atoms all of which are in ground stat...
Text Solution
|
- Let A(0) be the area enclined by the orbit in a hydrogen atom .The gra...
Text Solution
|
- In the hydrogen atom, an electron makes a transition from n=2 to n=1....
Text Solution
|
- A stationary hydrogen atom emits photon corresponding to the first lin...
Text Solution
|
- Light wave described by the equation 200 V//m sin (1.5xx10^15 s^(-1)...
Text Solution
|
- A hydrogne like atom is excited using a radiation . Consequently, six...
Text Solution
|
- The time period of the electron in the ground state of hydrogen atom i...
Text Solution
|
- The wavelengths of Kalpha X-rays from lead isotopes Pb^(204) , Pb^(20...
Text Solution
|
- in cases of hydrogen atom, whenever a photon is emitted in the Balmer ...
Text Solution
|
- An electron of kinetic enregy K collides elastically with a stationary...
Text Solution
|
- In a stationary hydrogen atom, an electron jumps from n = 3 ot n =1. ...
Text Solution
|
- An X-ray tube is operating at 150 kV and 10 mA. If only 1% of the ele...
Text Solution
|
- An electron revolves round a nucleus of atomic number Z. if 32.4 eV of...
Text Solution
|
- If the de-Broglie wavelength of a proton is 10^(-13) m, the electric p...
Text Solution
|
- If En and Ln denote the total energy and the angular momentum of an el...
Text Solution
|
- An orbital electron is the ground state of hydrogen has the magnetic ...
Text Solution
|
- A moving hydrogen atom makes a head on collision with a stationary hy...
Text Solution
|
- In an excited state of hydrogen like atom an electron has total energy...
Text Solution
|
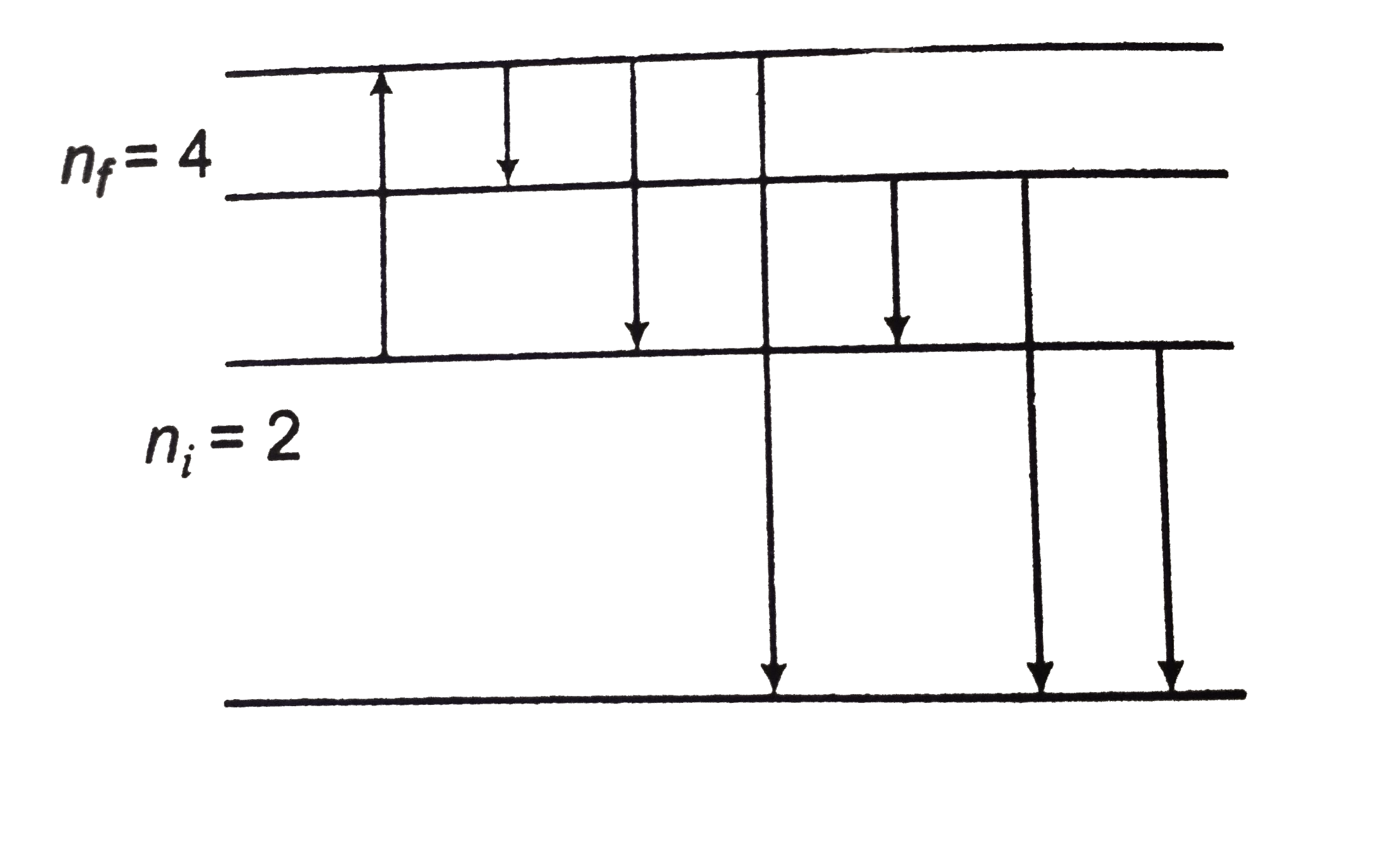 From `n_i =2`, energy of six emission lines is either
From `n_i =2`, energy of six emission lines is either