A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
MODERN PHYSICS - 1
DC PANDEY|Exercise Level 2 Comprehension Based|3 VideosMODERN PHYSICS - 1
DC PANDEY|Exercise Level 2Passage 2|1 VideosMODERN PHYSICS - 1
DC PANDEY|Exercise Level 2 Single Correct|22 VideosMODERN PHYSICS
DC PANDEY|Exercise Integer Type Questions|17 VideosMODERN PHYSICS - 2
DC PANDEY|Exercise Level 2 Subjective|10 Videos
Similar Questions
Explore conceptually related problems
DC PANDEY-MODERN PHYSICS - 1-Level 2 More Than One Correct
- If the potential difference of coolidge tube probucing X-ray is increa...
Text Solution
|
- In Bohr model of the hydrogen atom, let R,v and E represent the radiu...
Text Solution
|
- The magnitude of angular momentum, orbital radius and time period of r...
Text Solution
|
- In which of the following cases the havier of the two particles has a ...
Text Solution
|
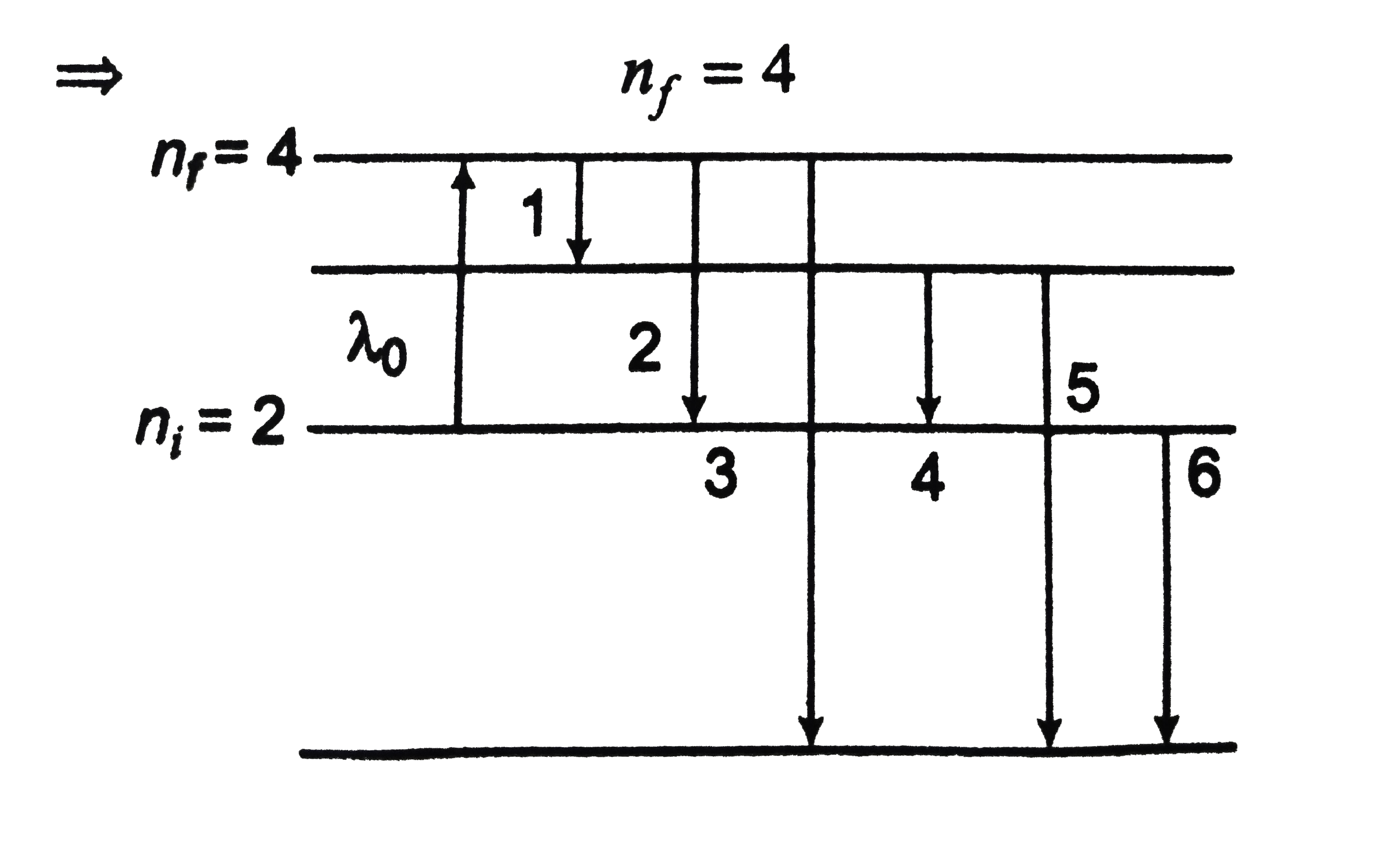
- Hydrogen atom absorbs radiations of wavelength lambda0 and consequentl...
Text Solution
|
- In coolidge tube, if f and lambda represent the frequency and waveleng...
Text Solution
|