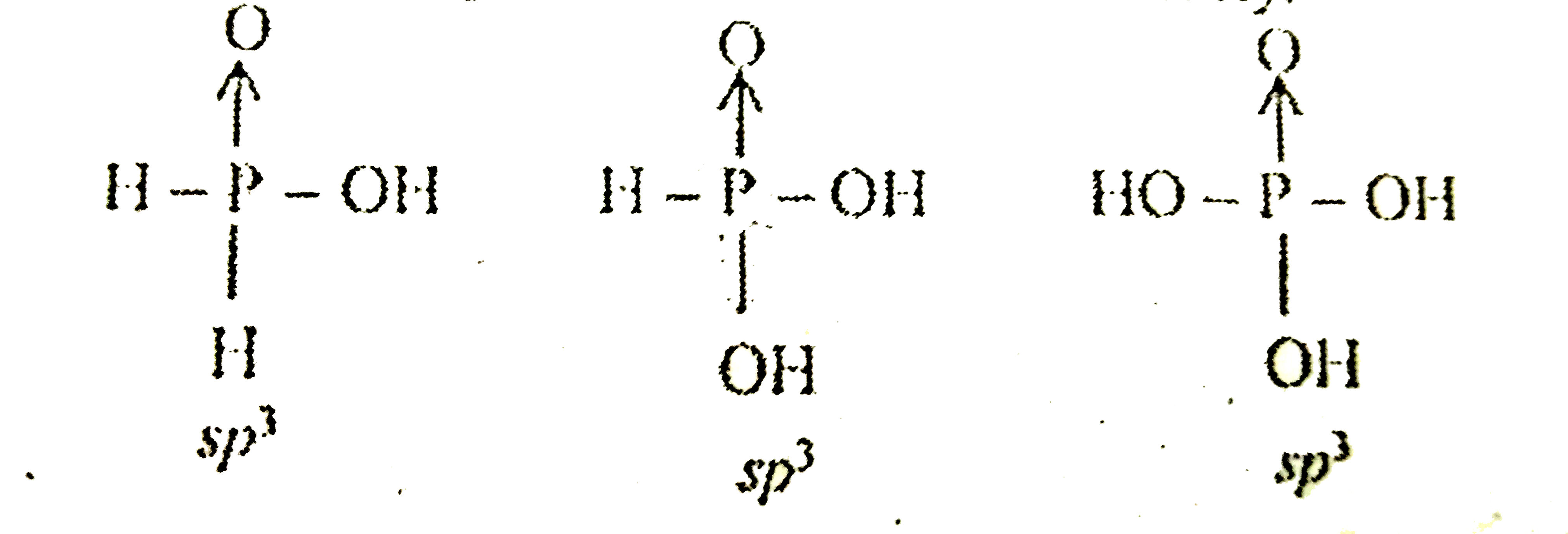A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
AIIMS PREVIOUS YEAR PAPERS-AIIMS 2003-CHEMISTRY
- The paramagnetic species is
Text Solution
|
- The reagent commonly used to determine hardness of water titrimetrical...
Text Solution
|
- The true statement of the acids of phosphorus H3 PO2, H3 PO2 and H3 PO...
Text Solution
|
- The ion which is not tetrahedral in shape is
Text Solution
|
- The complex used as an anticancer agent is
Text Solution
|
- The colourless species is
Text Solution
|
- MnO(4)^(2-) (1 mole) in neutral aqueous medium is disproportionate to
Text Solution
|
- Lanthanide for which +II and +III oxidation states are common is
Text Solution
|
- The mixture of concentrated HCl and HNO(3) made in 3:1 ratio contains
Text Solution
|
- On dissolving moderate amount of sodium metal in liquid ammonia at low...
Text Solution
|
- The ligand called pi acid is
Text Solution
|
- The compound used for gravimetric estimation of Cu(II) is:
Text Solution
|
- In the extraction of Cu from its sulphide ore, the metal is formed by ...
Text Solution
|
- Among the following the strongest acid is
Text Solution
|
- Among the following the weakest base is .
Text Solution
|
- IUPAC name of is
Text Solution
|
- Intermolecular hydrogen bonding is strongest in
Text Solution
|
- The ortho/para directing group among the following is
Text Solution
|
- The treatment of benzene with isobutene in the presence of sulphuric a...
Text Solution
|
- Which of the following alkoxides is the most reactive nucleophile?
Text Solution
|