A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
AIIMS PREVIOUS YEAR PAPERS-AIIMS 2004-Chemistry
- Which one of the following forms with an excess of CN^(-) (Cyanide) a ...
Text Solution
|
- Which of the following is not considered as an organometallic compound...
Text Solution
|
- Dimethyl glyoxime gives a red precipitate with Ni^(2+), which is used ...
Text Solution
|
- The element which forms oxides in all oxidation states +1 to +5 is.
Text Solution
|
- For decolourisation of 1 "mol of" KMnO(4), the moles of H(2)O(2) requi...
Text Solution
|
- The statement true for N(3)^(-) is
Text Solution
|
- Which of the following does not have optical isomer?
Text Solution
|
- For the electron affinity of halogens (with -ve sign), which of the fo...
Text Solution
|
- Shape of O(2)F(2) is similar to that of
Text Solution
|
- The liquified metal expanding on solidification is :
Text Solution
|
- The compound insoluble in water is
Text Solution
|
- Which of the following imparts colour to the burner flame?
Text Solution
|
- The ONO bond angle is maximum in
Text Solution
|
- Among the following the dissociation constant is highest for
Text Solution
|
- The strongest base among the following .
Text Solution
|
- The compound having only primary hydrogen atoms is
Text Solution
|
- Among the following, the aromatic compound is
Text Solution
|
- The dipole moment is the highest for
Text Solution
|
- The geometrical isomerism is shown by:
Text Solution
|
- The reagent used for the separation of Acetadehyde from acetophenone i...
Text Solution
|
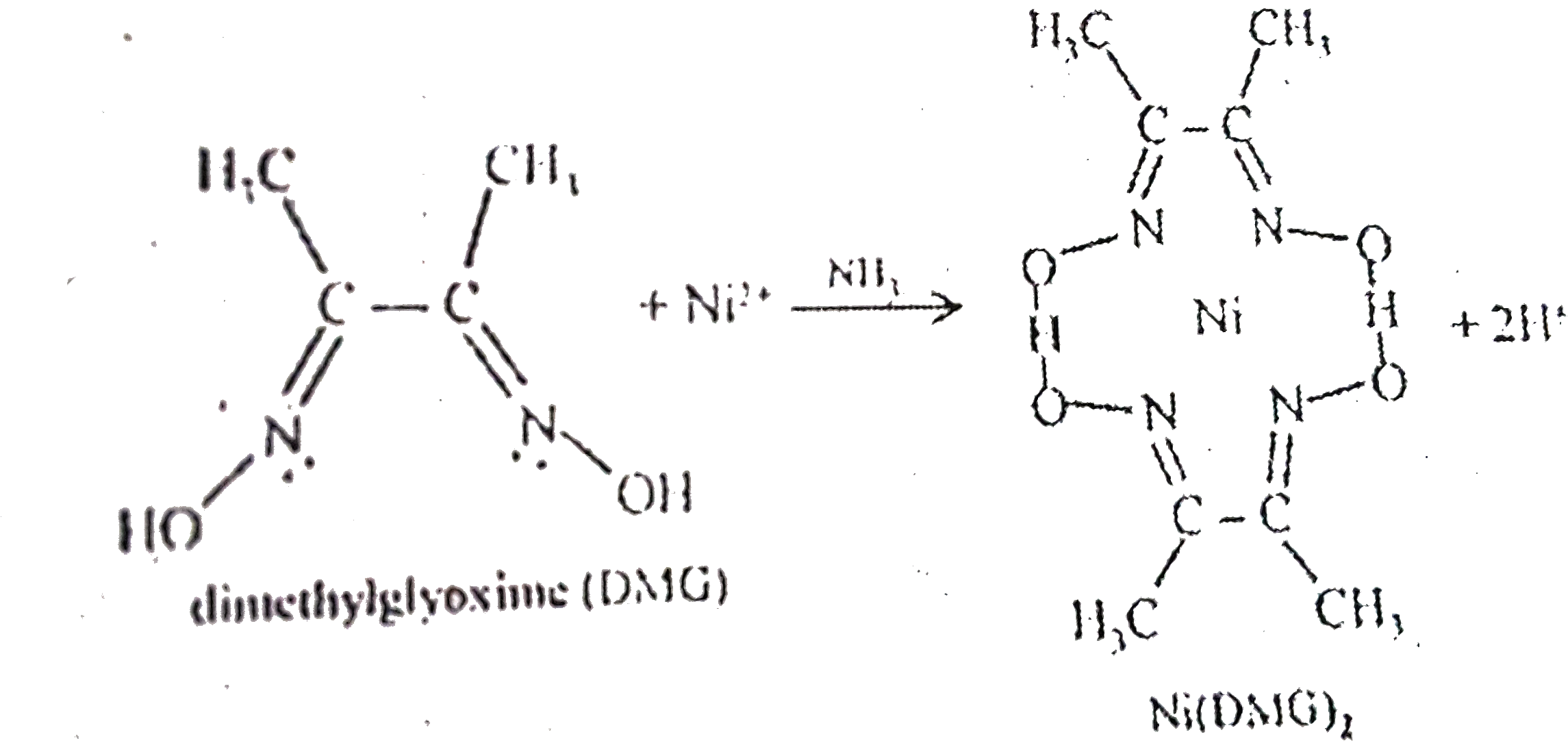 Although the does loss of one proton occurs from one oxime group(NOH) on each of two molecules of dimethyglyoxime,the chelation reaction occurs due to donation of the electron pairs on the oxygen atoms.The reaction is not by electrons on the oxygen atoms.The reaction is performed in a solution buffered by either an ammonia or citrate buffer to prevent the pH do-es become too low the equillibrium of the above reaction favors the formation of nickel(II) ion,causing the dissolution of `Ni(DMG)_(2)` back into the mother liquor.
Although the does loss of one proton occurs from one oxime group(NOH) on each of two molecules of dimethyglyoxime,the chelation reaction occurs due to donation of the electron pairs on the oxygen atoms.The reaction is not by electrons on the oxygen atoms.The reaction is performed in a solution buffered by either an ammonia or citrate buffer to prevent the pH do-es become too low the equillibrium of the above reaction favors the formation of nickel(II) ion,causing the dissolution of `Ni(DMG)_(2)` back into the mother liquor.