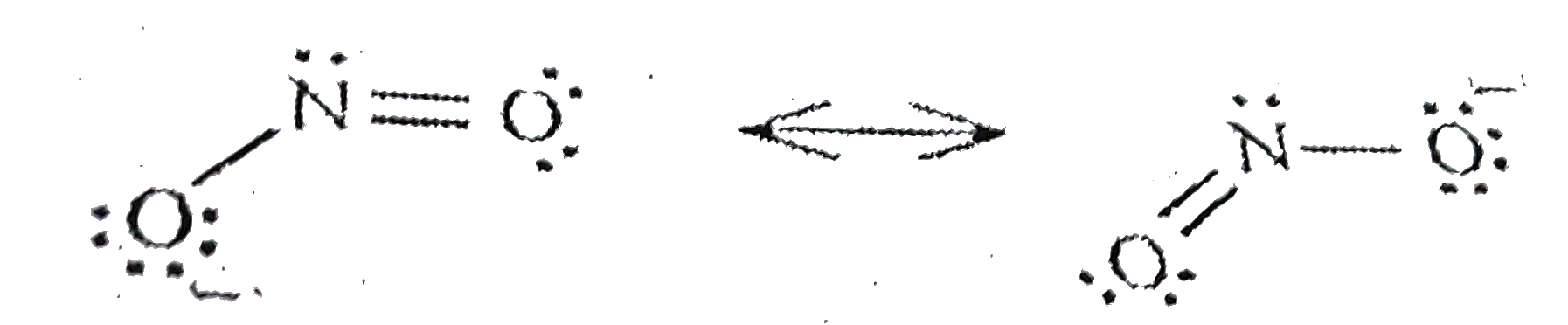
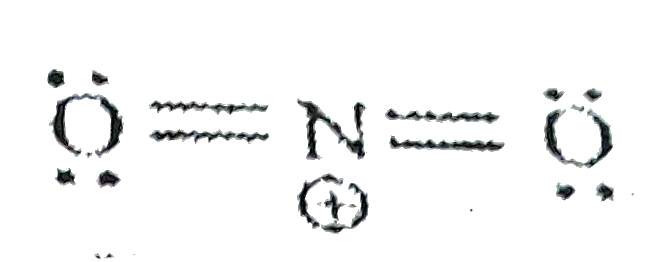
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
AIIMS PREVIOUS YEAR PAPERS-AIIMS 2004-Chemistry
- The compound insoluble in water is
Text Solution
|
- Which of the following imparts colour to the burner flame?
Text Solution
|
- The ONO bond angle is maximum in
Text Solution
|
- Among the following the dissociation constant is highest for
Text Solution
|
- The strongest base among the following .
Text Solution
|
- The compound having only primary hydrogen atoms is
Text Solution
|
- Among the following, the aromatic compound is
Text Solution
|
- The dipole moment is the highest for
Text Solution
|
- The geometrical isomerism is shown by:
Text Solution
|
- The reagent used for the separation of Acetadehyde from acetophenone i...
Text Solution
|
- Among the following,the most reactive towards alcoholic KOH is
Text Solution
|
- Among the following, one which reacts most readily with ethanol is
Text Solution
|
- The nucleic acid base having two possible binding sites is:
Text Solution
|
- alpha-Toluic acid in reaction withBr(2)+Fe gives
Text Solution
|
- Aromatic nitriles(ArCN) are not prepared by reaction:
Text Solution
|
- Melting points are normally highest for
Text Solution
|
- The most suitable reagent for the conversion of RCH(2)OHtoRCHO is
Text Solution
|
- Which of the following is arranged in the increasing order of enthalpy...
Text Solution
|
- For principle quantum number n=4,the total number of orbitals having l...
Text Solution
|
- The average osmotic pressure of humna blood is 7.8 bar at 37^(@)C.What...
Text Solution
|
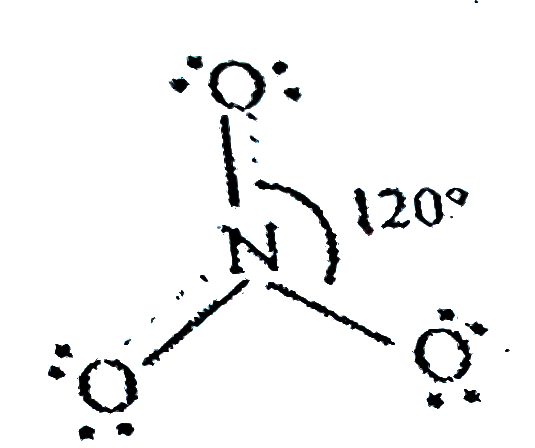 `NO_(2)^(-)` 18 electrons,ideal geometry trigonal planer,`sp^(2)`with bond angle of`116^(@)`
`NO_(2)^(-)` 18 electrons,ideal geometry trigonal planer,`sp^(2)`with bond angle of`116^(@)`