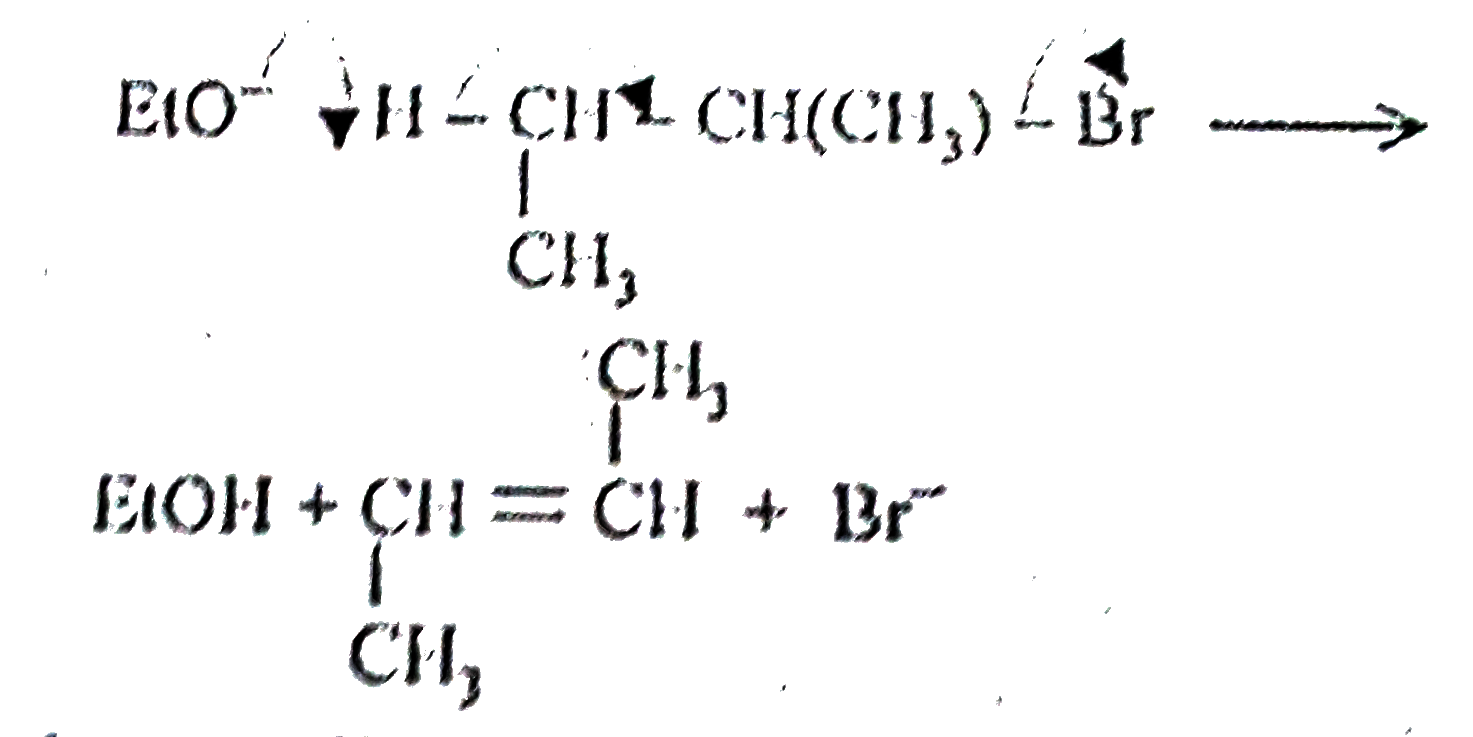A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
AIIMS PREVIOUS YEAR PAPERS-AIIMS 2004-Chemistry
- Assertion:HClO(4) is a stronger acid than HClO(3) :Oxidation state of ...
Text Solution
|
- The free gaseous Cr atom has six unpaired electrons. Half-filled s-o...
Text Solution
|
- Assertion: [Ni(en)(3)]Cl(2) has lower stability than [Ni(NH(3))(6)]Cl(...
Text Solution
|
- Assertion:Sb(III) is not precipitated as sulphide when in its alkaline...
Text Solution
|
- Assertion:Nuclear binding energy per nucleon is in the order -""(4)^9B...
Text Solution
|
- Assertion (A): magnesium is not present in enamel of human teeth. Re...
Text Solution
|
- Assertion. Carboxypetidase is an exopeptidase. Reason. It cleaves th...
Text Solution
|
- Assertion : Sucrose is a non- reducing sugar. Reason : It has glycos...
Text Solution
|
- Assertion:Isobutanal does not give iodoform test. Reason:It does not h...
Text Solution
|
- Assertion:Styrene on reaction with HBr gives 2-bromo-2-phenylethane. ...
Text Solution
|
- Assertion:The pK(a) of acetic acid is lower than that of phenol. Reaso...
Text Solution
|
- Assertion:2-Bromobutane on reaction with sodium ethoxide in ethanol gi...
Text Solution
|
- Assertion:The major products formed by heatingC(6)H(5)CH(2)OCH(3) with...
Text Solution
|
- Assertion: Molar entropy of vaporization of water is different from et...
Text Solution
|
- Assertion: A quious gold colloidal solution is red in colour. Reason...
Text Solution
|
- Assertion (A) : Cu gets readily corroded in acidic aqueous solution. ...
Text Solution
|
- Assertion:Addition of silver ions to a mixture of aqueos sodium chlori...
Text Solution
|
- Assertion: Alcohols are dehydrated to hydrocarbons in the presence of ...
Text Solution
|
- Assertion : All F - S - F angle in SF(4) are greater than 90^(@) but ...
Text Solution
|
- Assertion: Effusion rate of oxygen is smaller than nitrogen. Reason:...
Text Solution
|